Environmentally-friendly preparation method of ciprofibrate
An environment-friendly, dichlorocyclopropyl-based technology, applied in the field of environment-friendly preparation of ciprofibrate, can solve problems such as unsuitability for large-scale industrial production, potential safety hazards or pollution, and achieve mild conditions and high safety Sexuality and easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
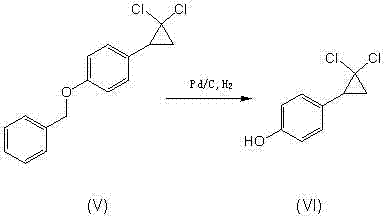
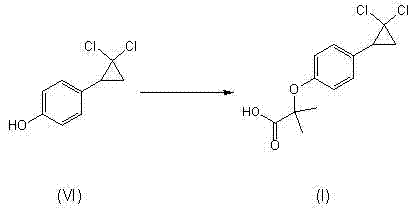
Image
Examples
Embodiment 1
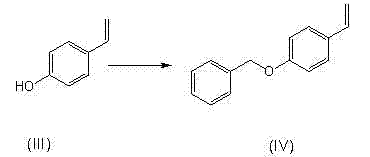
[0066] 1) Preparation of p-hydroxystyrene from p-hydroxybenzaldehyde
[0067] Add 833g of p-hydroxybenzaldehyde and 2500ml of pyridine into a 10L flask, weigh 1450g of malonic acid, add to the reaction flask in batches and stir, add dropwise 140ml of piperidine, finish adding in 30min, and then reflux until the reaction is complete. The solvent was spin-dried, and 4000ml of water and 3000ml of dichloromethane were added, and the aqueous layer was washed with 2000ml of dichloromethane, and the organic phases were combined, washed with 7000ml of 10% citric acid aqueous solution, dried with anhydrous sodium sulfate, and distilled under reduced pressure to obtain 675g of the product. The molar yield is 83%.
[0068] 2) Preparation of 1-benzyloxy-4-styrene from p-hydroxystyrene
[0069] Dissolve 277g p-hydroxystyrene in 1000ml acetone, add 586g K 2 CO 3 , Add 351g of benzyl chloride dropwise, then heat the reaction to 57°C, reflux until the reaction is complete, and filter. ...
Embodiment 2
[0077] 1) Preparation of p-hydroxystyrene from p-hydroxybenzaldehyde
[0078] Add 400g of p-hydroxybenzaldehyde and 1000ml of pyridine to a 10L flask, weigh 681g of malonic acid, add to the reaction flask in batches, stir, and heat to reflux until the reaction is complete. Recover the solvent under reduced pressure, add 2000ml of water and 2000ml of dichloromethane, wash the aqueous layer with 2000ml of dichloromethane, combine the organic phases, then wash with 2000ml of 10% aqueous hydrochloric acid, dry with anhydrous sodium sulfate, and distill under reduced pressure to obtain 330g of the product. The molar yield is 84.1%.
[0079] 2) Preparation of 4-(methoxymethoxy)styrene from p-hydroxystyrene
[0080] Dissolve 200g of p-hydroxystyrene in 500ml of tetrahydrofuran, lower the temperature to 0°C, add 73.3g of 60% NaOH in batches, and after stirring for half an hour, add 141g of chloromethyl methyl ether dropwise, raise the temperature to 20-25°C, and stir until The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com