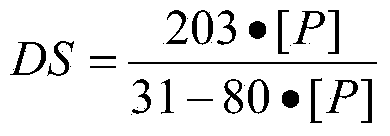Method for synthesizing phosphorylated chitin by using methanesulfonic acid as solvent
A technology of methanesulfonic acid and chitin, applied in the field of biochemical industry, can solve the problems of restricting wide application and achieve the effects of long process route, high yield and new physiological and biochemical activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
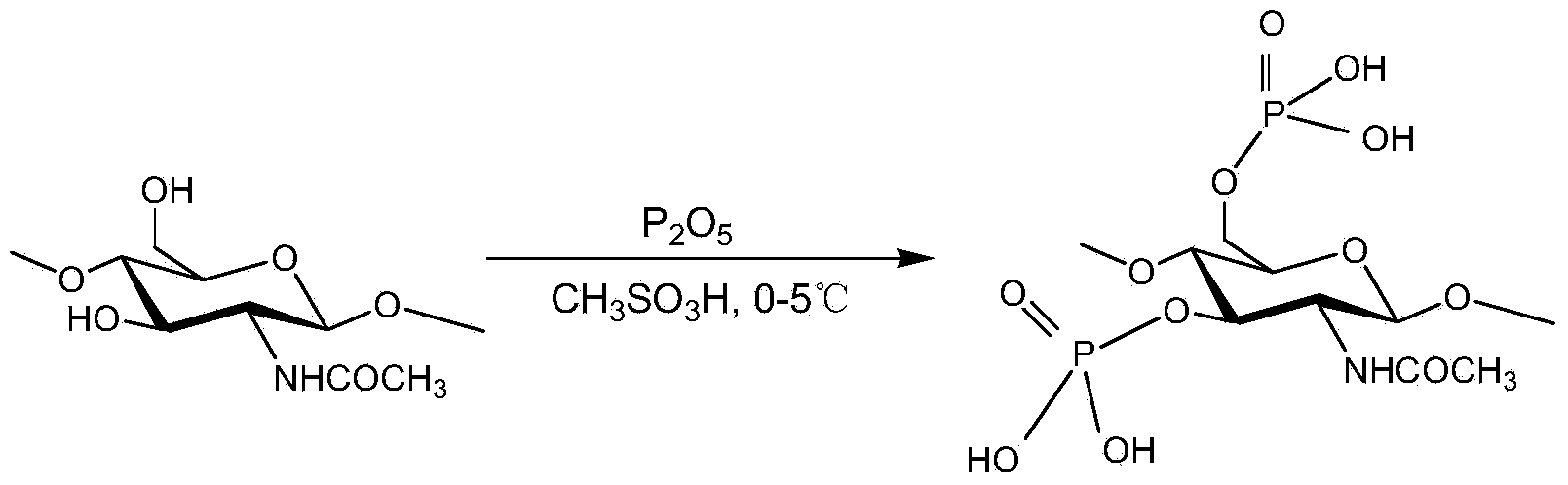
[0028] Preparation of Eaton reagent: Methanesulfonic acid is placed in a flask; phosphorus pentoxide is weighed in a dry state and added to the flask, and mixed with methanesulfonic acid for 1.5 hours under constant stirring; methylsulfonic acid and phosphorus pentoxide The mass ratio is 10:1;
[0029] Reaction: Grind the dried chitin, pass through a 100-mesh sieve, mix 2 g of chitin that passed through the sieve with 14 ml of Eaton reagent (14 g), and continue to stir and react at 2°C for 2 hours under the protection of nitrogen. Add 70ml of ether to precipitate and centrifuge. Then wash with 42ml acetone and 42ml methanol successively. The precipitate was then dissolved with 50 ml of deionized water. The turbid liquid was dialyzed with a molecular weight cut-off of 3500, then concentrated in vacuum, and the concentrated liquid was freeze-dried to obtain the product. The measured substitution degree of phosphorylated chitin was 0.71.
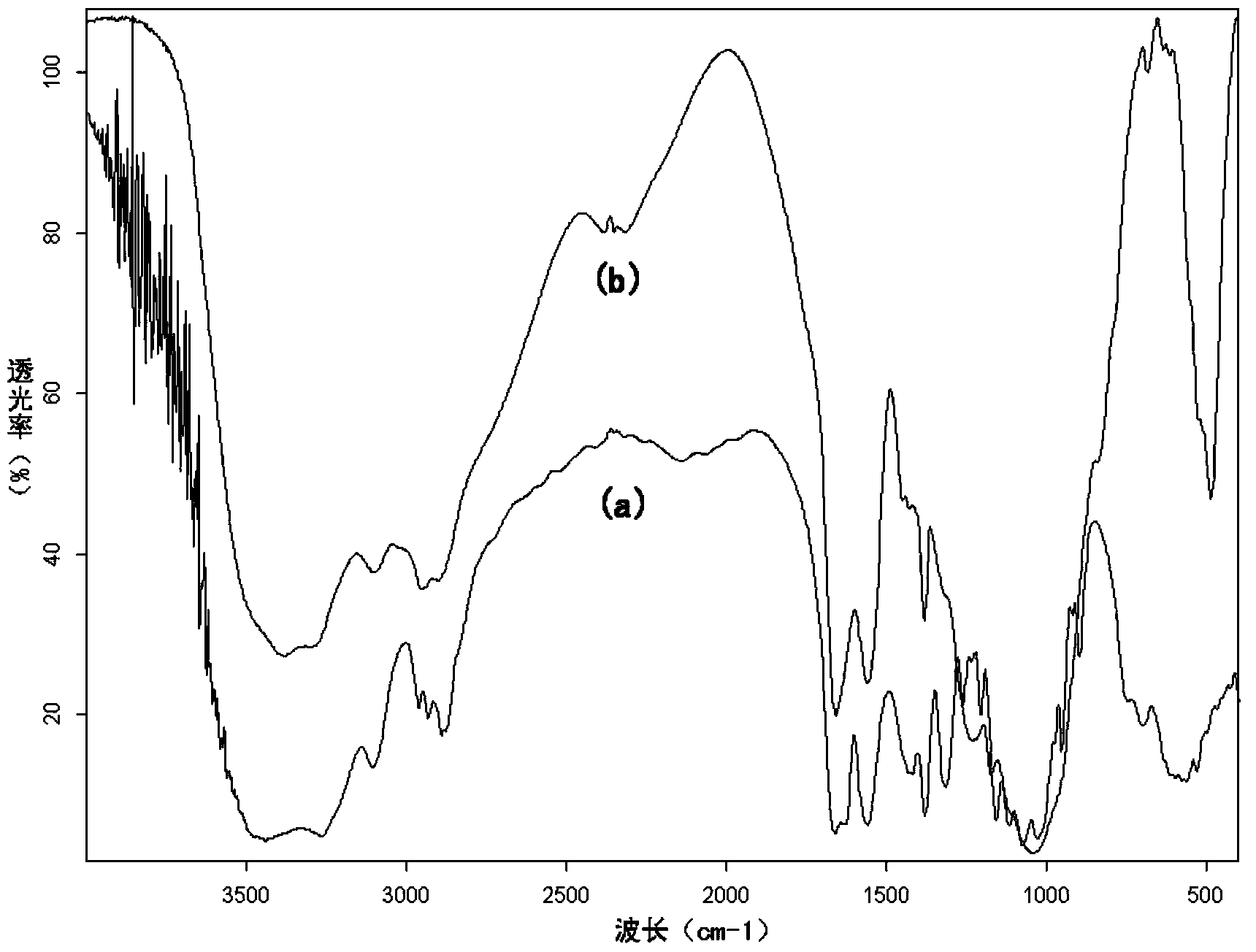
[0030] figure 1 (a) is the infrared...
Embodiment 2
[0033] Preparation of Eaton reagent: Methanesulfonic acid is placed in a flask; phosphorus pentoxide is weighed in a dry state and added to the flask, and mixed with methanesulfonic acid for 1.5 hours under constant stirring; methylsulfonic acid and phosphorus pentoxide The mass ratio is 10:1;
[0034] Reaction: crush the dried chitin, pass through a 100-mesh sieve, mix 3g of chitin that has passed through the sieve with 20ml of Eaton reagent (20g), and stir continuously at 4°C under the protection of nitrogen, and slowly add phosphorus pentoxide 1.5g, reacted for 3 hours. Add 100ml ether to precipitate and centrifuge. Then wash with 60ml acetone and 60ml methanol successively. Dissolve the precipitate with 80ml of deionized water. The turbid liquid was dialyzed with a molecular weight cut-off of 6000, then concentrated in vacuum, and the concentrated liquid was freeze-dried to obtain the product. The degree of substitution of phosphorylated chitin was measured to be 1.15....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com