Laccase mutant and encoding gene and application thereof
A coding and gene technology, applied in the field of genetic engineering, can solve the problems of poor stability, low specific activity, and expensive price, and achieve the effect of high specific activity and wide range of pH tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the cloning of wild-type laccase gene and the construction of recombinant engineering bacteria
[0044] 1. Cloning of POXA1b gene of laccase from Pleurotus florida ACCC No.50035
[0045] Specific steps for amplifying the laccase POXA1c gene from Pleurotus florida ACCC No.50035:
[0046]1. The total RNA of Pleurotus florida ACCC No.50035 was extracted with TRIZOL Total RNA Extraction Kit (Invitrogen).
[0047] 2. Take oligo-(dT) 15 (Promega) was used as a primer, and cDNA was synthesized by reverse transcription according to conventional methods.
[0048] 3. Use the following primers to amplify the POXa1b gene of laccase.
[0049] poxa1c-F:5'-CG GAATTC AGCATTGGGCCCCGCGGAACGCTG-3' (the underlined part is the recognition sequence of EcoR I, and the following sequence is the 1-24th position of sequence 8);
[0050] poxa1c-R: 5'-ATAGTTTA GCGGCCGC TCATGCTTTCAATGGCGCAGGCAG-3' (the underlined part is the recognition sequence of Not I, and the following seq...
Embodiment 2
[0060] Embodiment 2, the acquisition of laccase mutant gene and the construction of recombinant engineering bacteria
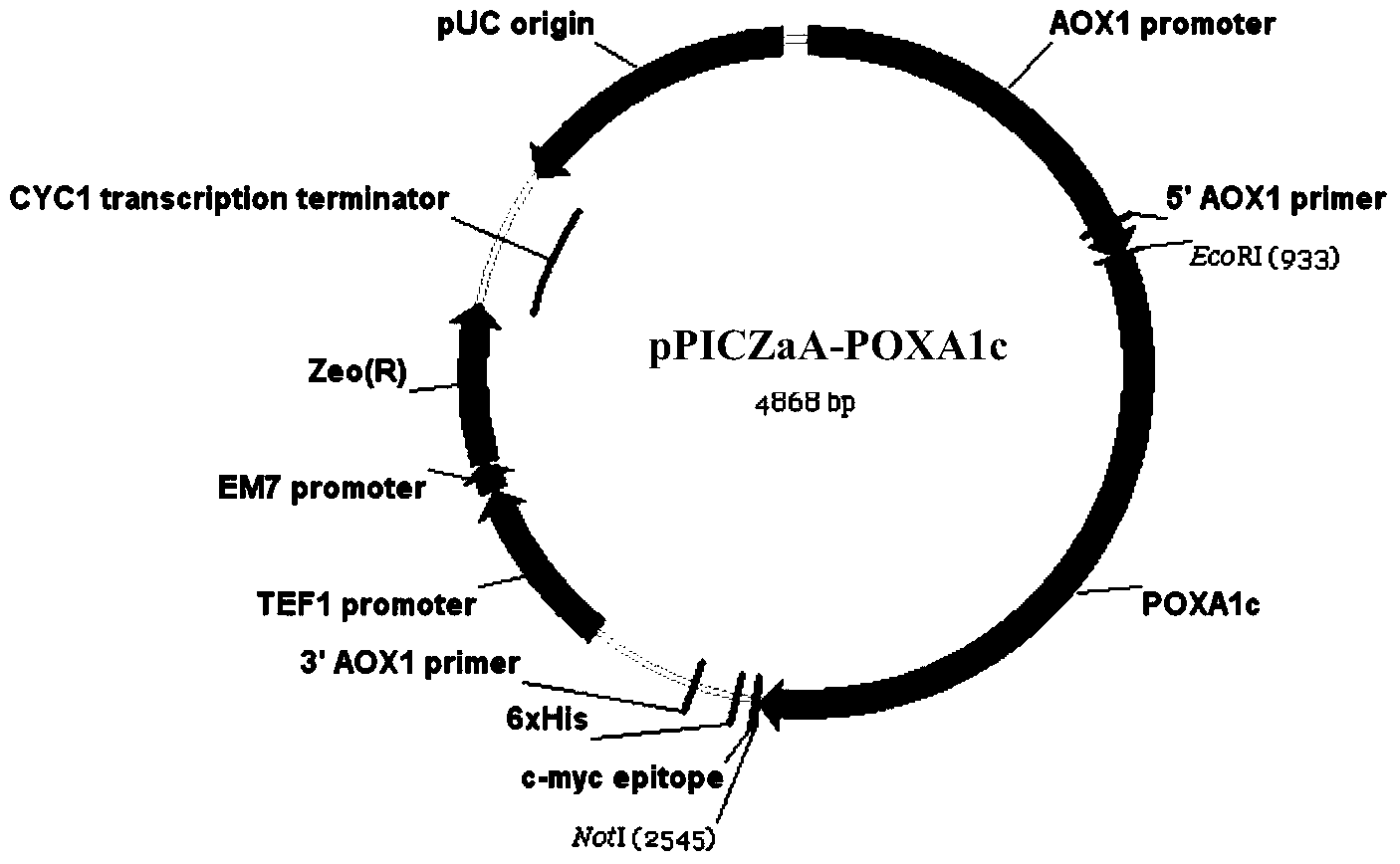
[0061] 1. Acquisition of laccase mutant POXA1c R5V coding gene and construction of recombinant expression vector
[0062] Using the recombinant expression vector pPICZαA-POXA1c obtained in Example 1 as a template, PCR amplification was performed using primers poxa1c R5V-F and poxa1c R5V-R.
[0063] poxa1c R5V-F: 5'-ATTGGGCCC GTT GGAACGCTGAACATCGCGAAC-3' (position 4-36 of sequence 6, the underlined part is the mutation site compared with sequence 8);
[0064] poxa1c R5V-R:5'-TTCC AAC GGGCCCAATGCT GAATTC -3' (the underlined part in italics is the recognition sequence of EcoR Ⅰ, the sequence before it is the reverse complementary sequence of the 1-19 position of sequence 6, and the underlined part is the mutation site compared with sequence 8).
[0065] The obtained PCR product was transformed into Escherichia coli competent DH5α, and the PCR product circu...
Embodiment 3
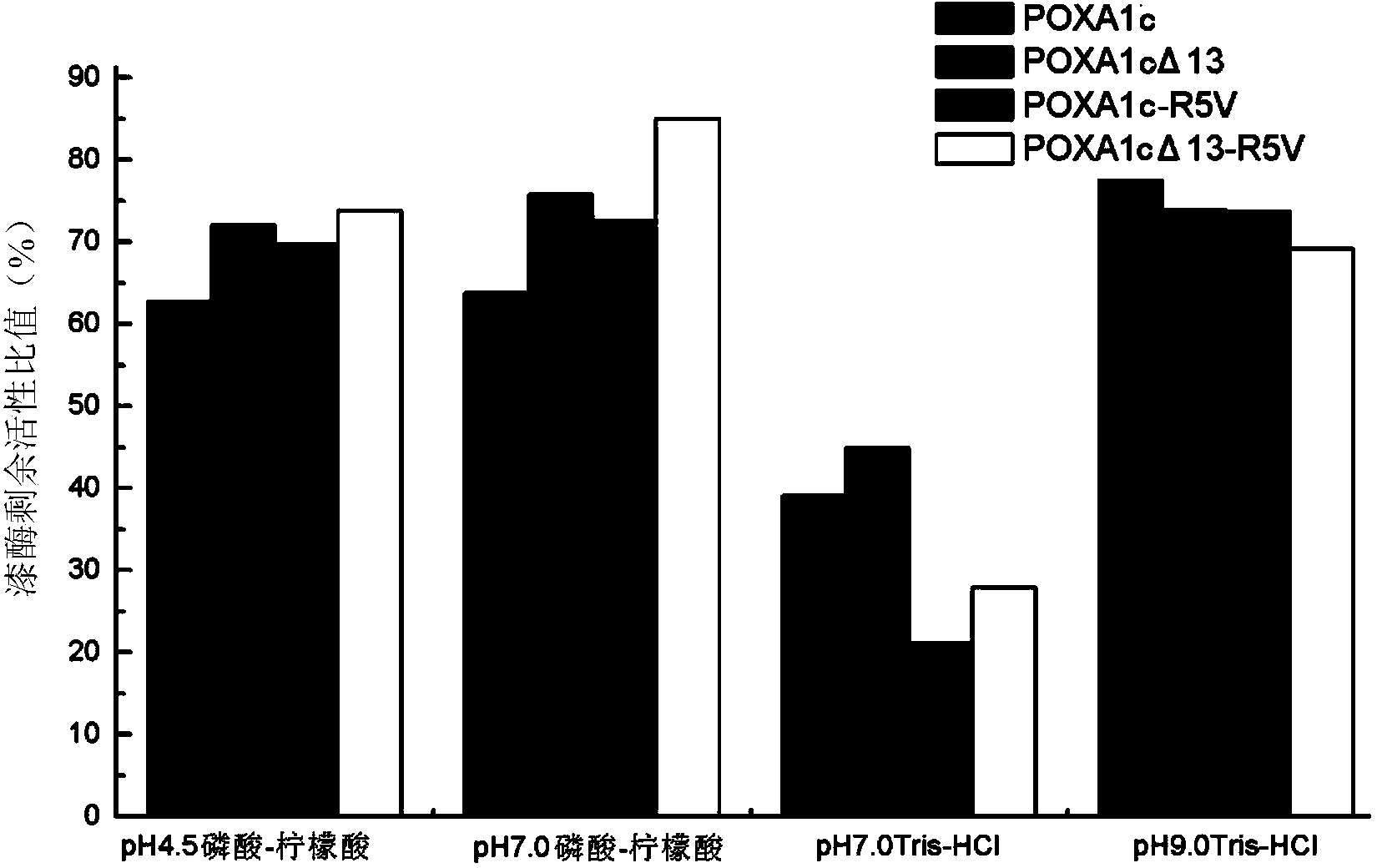
[0085] Embodiment 3, wild-type laccase and enzyme activity determination of different laccase mutants
[0086] 1. Fermentation of laccase-producing engineering strains, acquisition and purification of laccase
[0087] 1. Fermentation of laccase-producing engineering strains
[0088] The engineering bacteria X33 / pPICZαA-POXA1c expressing wild-type laccase POXA1c obtained in Example 1, the engineering bacteria X33 / pPICZαA-POXA1c R5V, X33 / pPICZαA-POXA1cΔ13-R5V and X33 expressing laccase mutants obtained in Example 2 / pPICZαA-POXA1cΔ13 were respectively cultured with fermentation medium, and after 24 hours of culture, anhydrous methanol was added to the fermentation system to induce the expression of laccase; the method of adding anhydrous methanol was once every 12 hours (the first The time point of the first addition is the 24th hour of cultivation), and a total of 5 additions were added, and anhydrous methanol was added each time to make the initial concentration of anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com