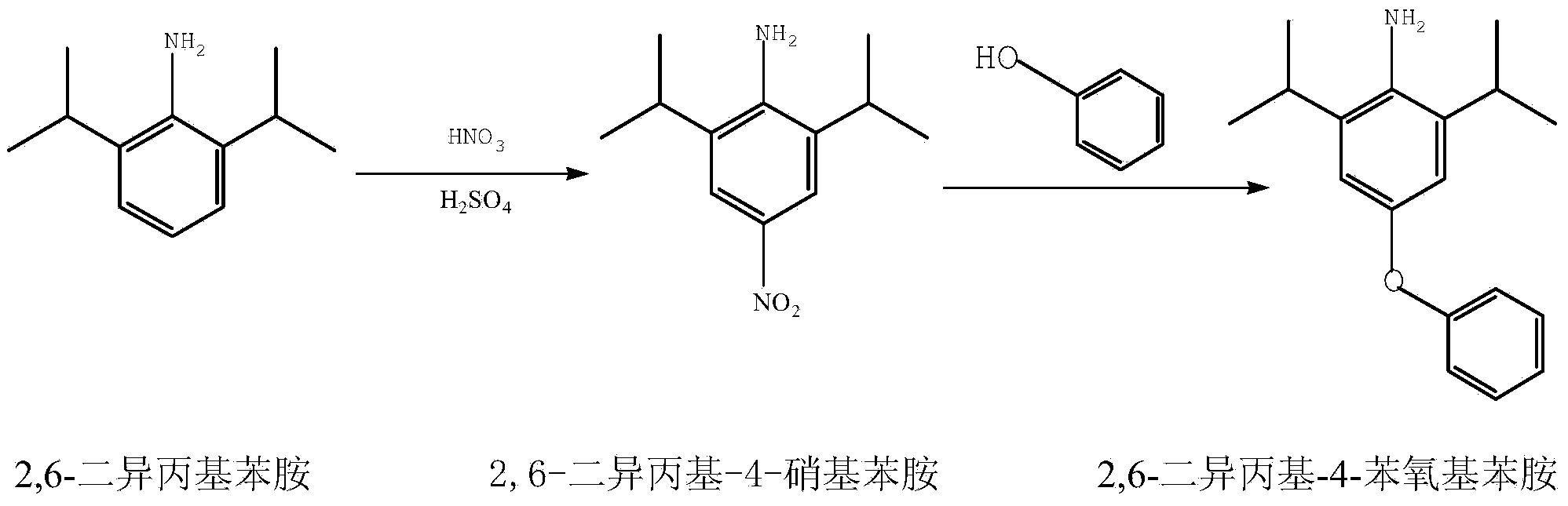
Synthetic method for 2,6-diisopropyl-4-phenoxy aniline
A technology of diisopropylaniline and phenoxyaniline, which is applied in the field of synthesis of 2,6-diisopropyl-4-phenoxyaniline, can solve the problem of high toxicity of brominated raw materials, which is unfavorable for large-scale production and equipment Harsh requirements and other issues, to achieve the effect of shortening the process cycle, benefiting environmental protection, and avoiding the discharge of waste acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 177.3g (1.0moL) of 2,6-diisopropylaniline, 600mL of toluene and 1.5g of sulfuric acid with a mass concentration of 98% in a 1000mL four-neck flask equipped with stirring, thermometer, reflux condensing device and dropping funnel, Raise the temperature to reflux temperature, slowly add 100.4g (1.1moL) of concentrated nitric acid with a mass concentration of 69wt%, control the reaction temperature at 110°C, keep it for 4h, and the reaction is over, then cool down to 70-80°C, add 373.3g (2moL) Potassium hydroxide with a concentration of 30wt%, 95g (1moL) phenol, and 0.9g catalyst tetrabutylammonium bromide were raised to reflux temperature to remove water in the reaction, and the reaction was completed at 110-112°C for 8 hours, and the organic phase was treated with 10wt% Wash with 150mL×3 sodium hydroxide, dry over anhydrous sodium sulfate, and then evaporate the solvent to obtain a brown solid. The crude product was recrystallized from ethanol to obtain 268.1g of pure...
Embodiment 2
[0029] Add 177.3g (1moL) of 2,6-diisopropylaniline, 600mL o-xylene and 1.5g (catalytic amount) to a 1000mL four-neck flask equipped with stirring, thermometer, reflux condensing device and dropping funnel. 98% sulfuric acid, heat up to 110-115°C, slowly add 109.6g (1.2moL) of concentrated nitric acid with a mass concentration of 68wt% dropwise, control the reaction temperature at 110-115°C, keep it warm for 4.5h, the reaction is over, and then cool down to 70- 80°C, add 373.3g (2moL) of potassium hydroxide with a mass concentration of 30wt%, 95g (1moL) of phenol, and 1.8g of catalyst tetrabutylammonium bromide, raise the temperature to reflux temperature, remove the water in the reaction, and react at 110°C for 8.5 At the end of h, the organic phase was washed with 10wt% sodium hydroxide 150mL×3, dried over anhydrous sodium sulfate, and then the solvent was distilled off to obtain a brown solid. The crude product was recrystallized from ethanol to obtain 268.8g of pure product ...
Embodiment 3
[0031] Add 177.3g (1moL) of 2,6-diisopropylaniline, 600mL toluene and 1.5g (catalytic amount) to a 1000mL four-necked flask equipped with a stirring, thermometer, reflux condensing device and a dropping funnel. The mass concentration is 98%. Sulfuric acid, heat up to reflux temperature, slowly add 105g (1.15moL) of concentrated nitric acid with a mass concentration of 69wt%, control the reaction temperature at 110-115°C, keep it warm for 4h, the reaction is over, then cool down to 70-80°C, add 266.7g (2moL) sodium hydroxide with a mass concentration of 30wt%, 95g (1moL) phenol, and 0.9g catalyst tetrabutylammonium iodide were heated to reflux temperature to remove water in the reaction, and the reaction was completed at 111°C for 8h, and the organic phase was treated with 10wt % sodium hydroxide 150mL×3, dried over anhydrous sodium sulfate, and evaporated the solvent to obtain a brown solid. The crude product was recrystallized from ethanol to obtain 269.0g of pure product, wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com