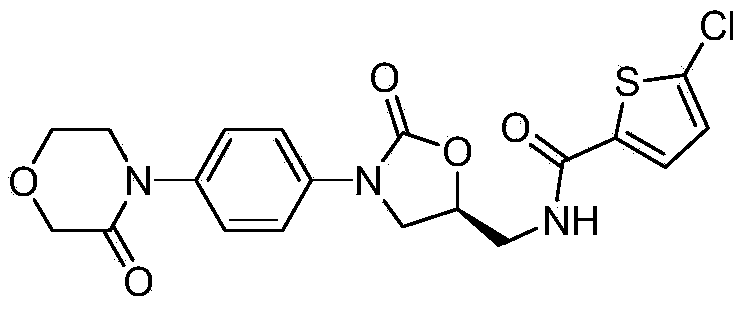
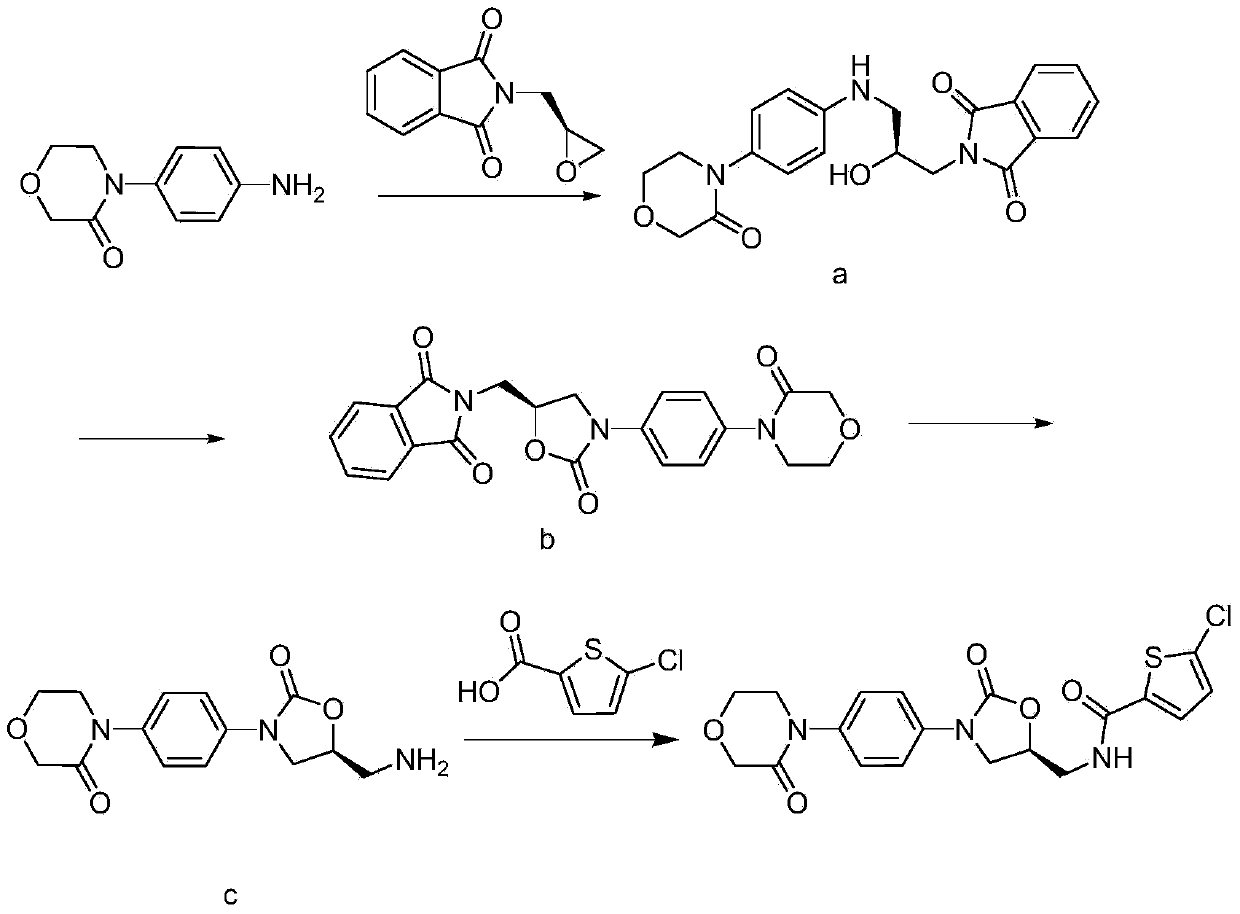
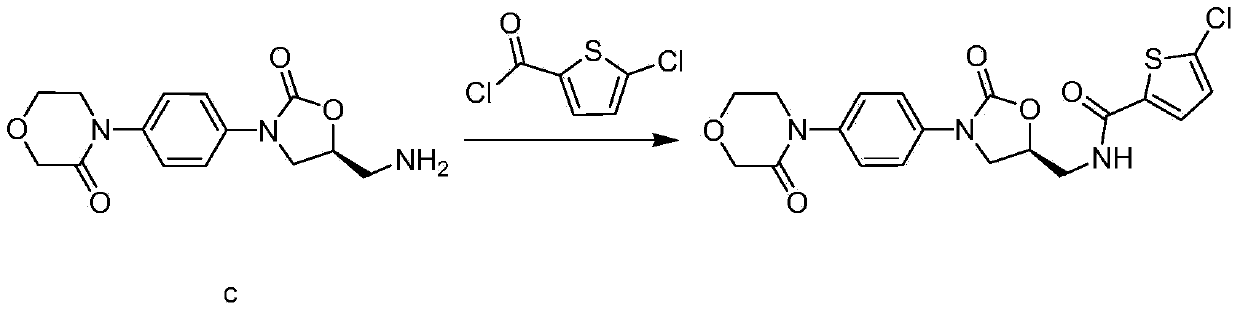
Synthesis method of novel anticoagulation drug
A synthetic method and technology of rivaroxaban, applied in the field of preparation of pharmaceutical compounds, can solve the problems of difficult production operation, low yield, corrosion equipment, etc., and achieve the effect of simple production operation, low price and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0059]
[0060] Add 5.4g 4-(4-aminophenyl)-3-morpholinone, 8.5g (S)-2-(2-oxiranylmethyl)-1H-isoindole-1 in 250mL reaction flask, 3-diketone, 140mL of a mixture of ethanol and water (V:V=9:1), heated and refluxed for 12h. Filter and wash the filter cake with ethanol. Dry to obtain 10.2 g of white solid, which is intermediate A, and the molar yield is 92% (based on 4-(4-aminophenyl)-3-morpholinone).
example 2
[0062]
[0063] Add 3.6g of intermediate a, 90mL of tetrahydrofuran, 2.9g of N,N-carbonyldiimidazole (CDI), and a catalytic amount of 4-dimethylaminopyridine (DMAP) into a 250ml reaction flask, raise the temperature to 60°C and stir for 24h, then evaporate under reduced pressure Solvent, to obtain a yellow solid, add 100ml of dichloromethane and 100ml of water, stir to dissolve and separate the liquids, the water layer is extracted once with 100ml of dichloromethane, the organic layer is combined, dried with anhydrous sodium sulfate and concentrated to dryness to obtain a white solid 3.3 g, is intermediate b, and the molar yield is 87% (based on intermediate A).
example 3
[0065]
[0066] Add 4.2g of intermediate b and 100mL of ethanol to a 250ml reaction flask, add dropwise 10.2mL of 40% methylamine aqueous solution, raise the temperature and reflux and stir for 1 hour, then evaporate the solvent to dryness under reduced pressure to obtain a white solid. Add 100ml of 50% hydrochloric acid ethanol solution, stir at room temperature for 4 hours, and filter to obtain 2.8g of white solid, which is morpholinone hydrochloride. The molar yield is 86% (based on intermediate B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com