Preparation method of Pralatrexate suitable for industrial large scale production
A synthetic method and industrial technology, applied in the field of preparation and purification of the antineoplastic drug pralatrexate raw material and its important intermediates, can solve the problems of difficult reaction and post-processing, low yield and purity, etc., and achieve the goal of reaction Post-processing is simple and feasible, with high purity and the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The present invention will be further described below by specific embodiment.
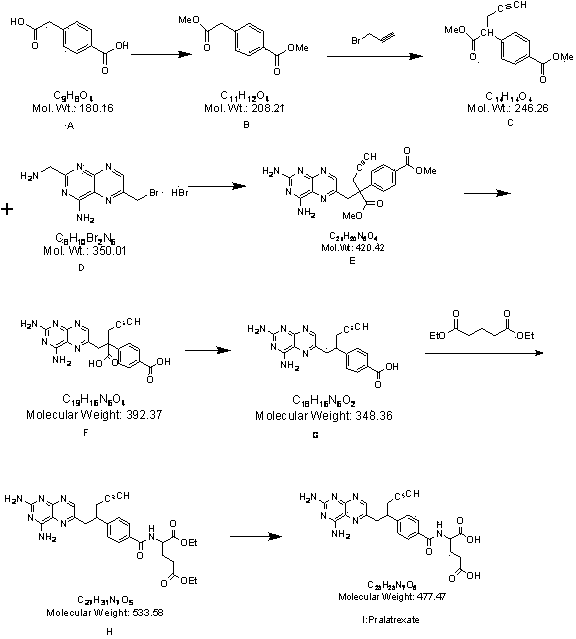
[0036] Synthesis of step 1 intermediate 4-methyl formate phenylacetate (B)
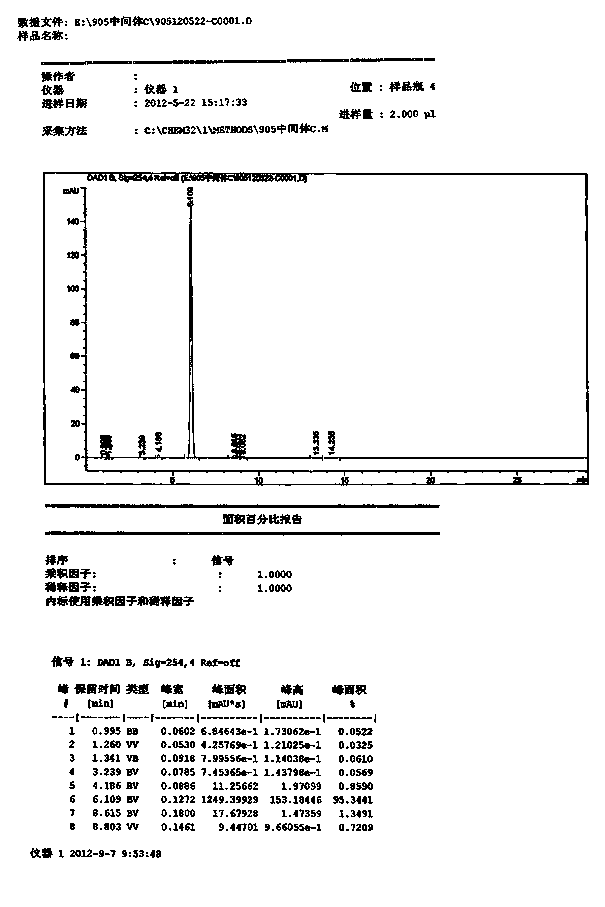
[0037]Add 80.0g of p-carboxyphenylacetic acid into a 2 L reaction kettle, stir and dissolve with 800 mL of anhydrous methanol, add 8.0g of p-toluenesulfonic acid, stir and heat under reflux for 24 h, spin out the methanol under reduced pressure, and add 240 mL of water to the residue , extracted with ethyl acetate (240mL×3), combined the organic phases, washed with 3% NaOH solution (80mL×2), and dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure and cooled to obtain Compound B as a white solid. Weight: 84.1g, yield: 91.1%, HPLC content: 97.8%.
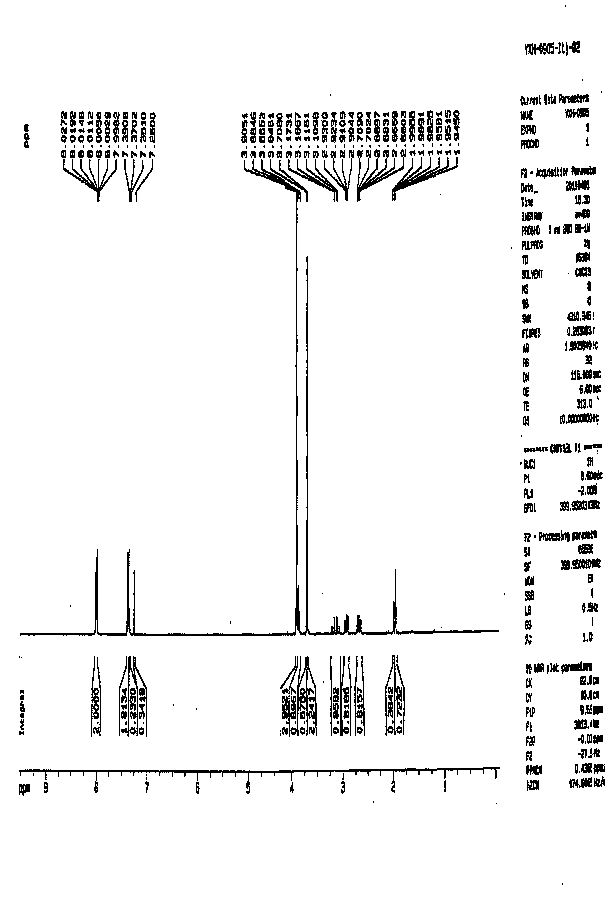
[0038] The H NMR spectrum data are as follows:
[0039] 1 H NMR (300 MHz, CDCl 3 )δ 7.91(d, J = 6.3 Hz, 2H), 7.42(d, J = 6.3 Hz, 2H), 3.84(s, 3H), 3.79(s, 2H), 3.62(s, 3H).
[0040] Step 2 Synthesis of intermediate ɑ-propargyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com