Method and equipment for preparing artemisinin by adopting dihydroarteannuic acid as raw material
A technology of dihydroartemisinic acid and artemisinin, applied in chemical instruments and methods, chemical/physical/physicochemical processes of energy application, chemical/physical/physicochemical processes, etc., can solve complicated operation and long reaction time and other problems, to achieve the effect of reducing the reaction temperature, low production cost and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
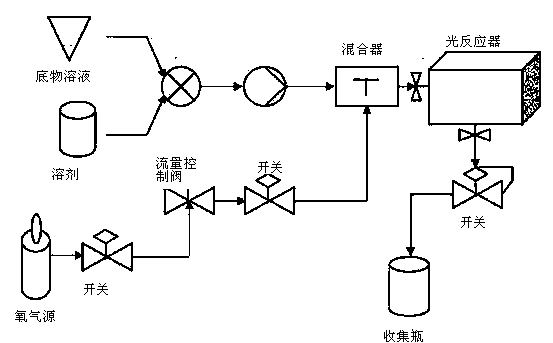
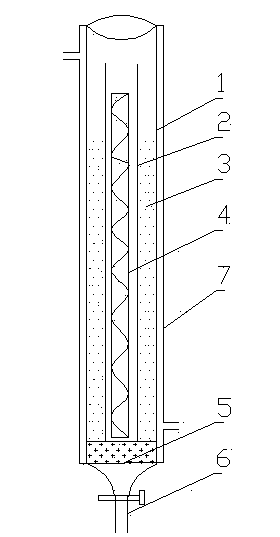
[0041] The dihydroartemisinic acid of 200g is dissolved in the dichloromethane of 1000mL, then pours in the photoreactor, then adds trifluoroacetic acid 80g and photosensitizer tetraphenylporphyrin 1g in the reactor; Reaction system is cooled to At -20°C, oxygen was introduced from the bottom port of the reactor through the sand core at a flow rate of 3L / min; half an hour later, the high-pressure mercury lamp was switched on, and the light reaction was carried out continuously for 4 hours at -20°C; then, the reaction system was The temperature was slowly raised to 20°C, stirred for 1 hour, and the light source was turned off.
[0042] Pour out the reaction solution, wash twice with 100 mL of saturated sodium bicarbonate solution, wash the organic phase with 50 mL of water, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a crude product; the crude product is reconstituted with a mixed solvent of ethanol and petroleum ether Crystallized to obtain 145g of art...
Embodiment 2
[0045] Dissolve 100g of dihydroartemisinic acid in 500mL of dichloromethane, then pour it into the photoreactor, then add 1g of photosensitizer rose bengal to the reactor, cool the reaction system to -50°C, Oxygen was introduced into the bottom interface through the sand core at a flow rate of 3L / min; after half an hour, the medium-pressure mercury lamp was switched on, and the light reaction was continued for 4 hours at -50°C; the light source was turned off, and then 40g of tris Fluoroacetic acid, and then continue to feed oxygen for 1 hour; then, slowly raise the temperature of the reaction system to 25° C., and react for 1 hour.
[0046] Pour out the reaction solution, wash twice with 50 mL of saturated sodium bicarbonate solution, then wash the organic phase with 30 mL of water, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a crude product; use a mixed solvent of ethanol and petroleum ether for the crude product Recrystallized to obtain 73g of artem...
Embodiment 3
[0048]Dissolve 200g of dihydroartemisinic acid in 1000mL of dichloromethane, then pour it into the photoreactor, and then add 1g of photosensitizer tetraphenylporphyrin to the reactor; cool the reaction system to -10°C, from the reaction Oxygen was introduced into the bottom of the device through the sand core at a flow rate of 3L / min; half an hour later, the deuterium lamp was switched on, and the light was continuously illuminated at -10°C for four hours.
[0049] Turn off the light source, pour out the reaction solution, quickly transfer it to a 2L three-neck flask, stir, and cool down to -20°C, feed oxygen at a flow rate of 3L / min, slowly add 80g of trifluoroacetic acid to the reaction system, and The reaction was carried out at 20° C. for 2 hours, and then the temperature of the reaction system was slowly raised to 25° C. and stirred for 1 hour.
[0050] Pour out the reaction solution, wash twice with 100 mL of saturated sodium bicarbonate solution, then wash the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com