Total organic carbon analysis instrument and method based on electrochemical catalytic oxidation
A technology of catalytic oxidation and total organic carbon, applied in the direction of material analysis, scientific instruments, and analytical materials through optical means, can solve problems such as high-temperature combustion, achieve the effects of improving measurement accuracy, mild reaction conditions, and increasing the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A boron-doped diamond electrode is used for the anode, and a stainless steel plate is used for the cathode; the boron-doped diamond electrode is obtained by chemical vapor deposition method, and a dense diamond film is deposited on the conductive tantalum surface by doping diborane in the gas phase.
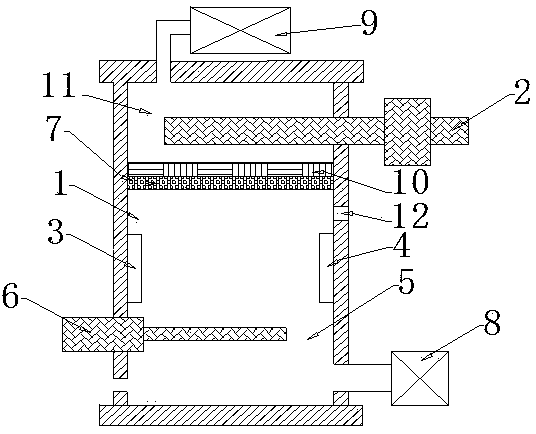
[0031] Inject the water sample into the electrochemical reaction cell 1, add NaH containing 99% 0.5 mol / L 2 PO 4 +1% 0.5 mol / L Na 2 HPO 4 Reaction solution 5, nitrogen gas was blown in to remove CO in gas phase and water phase 2 .
[0032] Determination of CO in gas phase and water phase by using non-dispersive infrared detector (NDIR) 2 and ion selective electrode 6 2 Concentration, the concentration of inorganic carbon (IC) in the solution is calculated.
[0033] Connect the cathode and anode to an external DC circuit, so that an electrochemical reaction occurs between the plates, and the organic carbon in the water is oxidized to CO 2 . Part of CO 2 Dissolved in...
Embodiment 2
[0038] The anode is made of graphite electrode, and the cathode is made of graphite electrode;
[0039] Inject the water sample into the electrochemical reaction cell 1, add NaH containing 99% 0.5 mol / L 2 PO 4 +1% 0.5 mol / L Na 2 HPO 4 The reaction solution 5 was blown with argon gas to remove CO in the gas phase and the water phase 2 .
[0040] Determination of CO in gas phase and water phase by using non-dispersive infrared detector (NDIR) 2 and ion selective electrode 6 2 Concentration, the concentration of inorganic carbon (IC) in the solution is calculated.
[0041] Connect the cathode and anode to an external DC circuit, so that an electrochemical reaction occurs between the plates, and the organic carbon in the water is oxidized to CO 2 . Part of CO 2 Dissolved in water, the other part enters the upper gas collection chamber 11 through the polypropylene gas diffusion membrane 7 and the moisture exchanger 10 made of TEFLON polymer tube layer material.
[0042] Th...
Embodiment 3
[0046] The anode is a platinum electrode, and the cathode is a boron-doped diamond electrode. The boron-doped diamond electrode is obtained by chemical vapor deposition, through gas-phase doping of diboron trioxide, and depositing a dense diamond film on the surface of conductive graphite.
[0047] Inject the water sample into the electrochemical reaction cell 1, add NaH containing 99% 0.5 mol / L 2 PO 4 +1% 0.5 mol / L Na 2 HPO 4 The reaction solution 5 was filled with helium gas to remove CO in the gas phase and water phase. 2 .
[0048] Determination of CO in gas phase and water phase by using non-dispersive infrared detector (NDIR) 2 and ion selective electrode 6 2 Concentration, the concentration of inorganic carbon (IC) in the solution is calculated.
[0049] Connect the cathode and anode to an external DC circuit, so that an electrochemical reaction occurs between the plates, and the organic carbon in the water is oxidized to CO 2 . Part of CO 2 Dissolved in water, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com