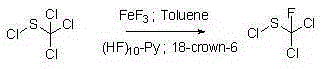Synthesis of n,n-dimethyl-n-phenyl-(n-fluorodichloromethylthio)-sulfonamide
A technology of fluorodichloromethylthio and fluorodichloromethylsulfanyl is applied in the field of synthesis technology of N,N-dimethyl-N-phenyl-sulfonamide, and can solve the problem of difficult industrial production of sulfonamide, The problems of high environmental hazard and high raw material cost have achieved the effect of simple post-processing, low environmental hazard, and reduced equipment condition requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of N,N-dimethyl-N-phenyl-(N-fluorodichloromethylthio)-sulfonamide:
[0054]
[0055] a. Add N,N-dimethyl-N'-phenylsulfonamide to toluene, add sodium hydride at 0-5°C, and react at room temperature for 0.5-1 hour;
[0056] b. Drop in a toluene solution with a mass concentration of 65-90% fluorodichloromethylsulfonyl chloride, and react at room temperature for 1-2 hours; N,N-dimethyl-N'-phenylsulfonamide and fluorodichloro The molar ratio of methylsulfonyl chloride is 1:0.8; the molar ratio of N,N-dimethyl-N'-phenylsulfonamide and sodium hydride is 1:1.0; N,N-dimethyl-N'-benzene The mass ratio of methyl sulfonamide and toluene is 1:6.0;
[0057] c. adding water to wash the reaction solution, and obtain an organic phase after liquid separation;
[0058] d. The organic phase is dried and concentrated to obtain the crude product of the product, and then refined to obtain the pure product of the product.
[0059] The mass ratio of crude N,N-dimethyl-N-phenyl-(...
Embodiment 2
[0062] The synthesis process of N,N-dimethyl-N-phenyl-(N-fluorodichloromethylthio)-sulfonamide is similar to Example 1, the difference is: N,N-dimethyl-N'-benzene The molar ratio of sulfonamide and fluorodichloromethylsulfonyl chloride is 1:1.4; the molar ratio of N,N-dimethyl-N'-phenylsulfonamide and sodium hydride is 1:1.2; N,N-di The mass ratio of methyl-N'-phenylsulfonamide to toluene is 1:8.65. The yield of N,N-dimethyl-N-phenyl-(N-fluorodichloromethylthio)-sulfonamide in this example was 84.2%. TLC: Pet:EA=5:1, Rf=0.5. 1 HNMR(CDCl3,300MHz)δ:2.76(s,6H,NCH3),7.36-7.44(m,3H),7.49-7.52(m,2H); 9 FNMR (CDCl3, 300MHz) δ: -27.30.
Embodiment 3
[0064] The synthesis process of N,N-dimethyl-N-phenyl-(N-fluorodichloromethylthio)-sulfonamide is similar to Example 1, the difference is: N,N-dimethyl-N'-benzene The molar ratio of sulfonamide and fluorodichloromethylsulfonyl chloride is 1:2.0; the molar ratio of N,N-dimethyl-N'-phenylsulfonamide and sodium hydride is 1:1.5; N,N-di The mass ratio of methyl-N'-phenylsulfonamide and toluene is 1:10. The yield of N,N-dimethyl-N-phenyl-(N-fluorodichloromethylthio)-sulfonamide in this example was 82.6%. TLC: Pet:EA=5:1, Rf=0.5. 1 HNMR (CDCl 3 ,300MHz)δ:2.78(s,6H,NCH 3 ),7.36-7.44(m,3H),7.49-7.52(m,2H); 9 FNMR (CDCl 3 ,300MHz) δ: -27.30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com