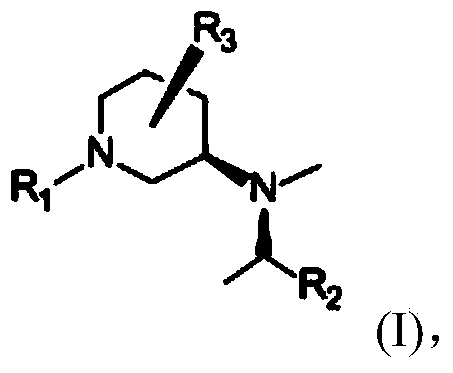
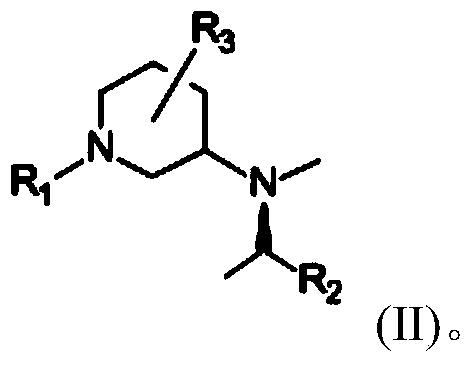
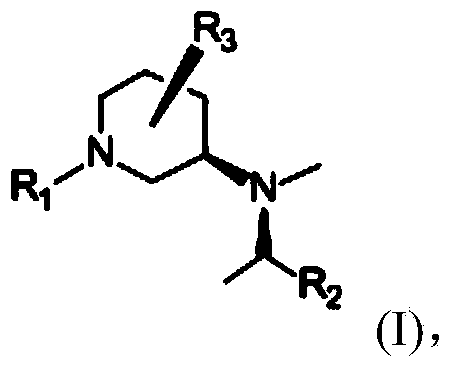
Synthetic method of piperidine derivative
A synthesis method and derivative technology, applied in bulk chemical production, carboxylate preparation, organic chemistry, etc., to achieve the effect of improving yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 1mol of 4-methyl-3-hydroxypyridine and 1.1mol of di-tert-butyl dicarbonate (Boc 2 O), heated to reflux for 10 hours, TLC showed that the reaction of the raw materials was complete, the reaction system was cooled to room temperature and diluted with ethanol, after cooling to 0°C, 0.9mol sodium borohydride was added, after the addition was completed, stirred at 0°C for 2 hours, TLC showed that the reaction was complete, Add 1mol / L hydrochloric acid aqueous solution, extract with methyl tert-butyl ether, and purify to obtain 1-tert-butoxycarbonyl-4-methyl-3-pyridone (0.86mol), yield 86%, structural formula:
[0029] 1 H NMR (300MHz, CDCl 3 ) 4.12(q,2H), 3.03-2.92(m,2H), 2.40(m,1H), 1.96-1.80(m,2H), 1.38(s,9H), 1.12(d,3H). MS:m / z=214(M+H) + .
Embodiment 2
[0031] 1.0mol of 1-tert-butoxycarbonyl-4-methyl-3-pyridone and 1.1mol of (S)-N-methyl-1-phenylethylamine (cas: 19131-99-8, ) was dissolved in 10 times the volume of dichloromethane (DCM). After stirring at 10°C for 1 hour, 3.2 mol of sodium acetate borohydride was added in batches and reacted for 4 hours. TLC showed that the reaction was complete. Water was added to separate layers and the organic phase was collected. , the obtained 1-tert-butoxycarbonyl-3-[(S)-N-methyl-1-phenylethylamino]-4-methyl-pyridine, structural formula: Ph represents a phenyl group. The purity of the product in the organic phase detected by high performance liquid chromatography is 91%, and the product yield is 95%. The collected organic phase does not need to be purified, and can be directly used for the next step reaction, LC-MS: m / z=333 (M+ h) + .
Embodiment 3
[0033] The ethanol solution containing the L-tartaric acid of 0.49mol is added dropwise in the organic phase adopting the method gain of embodiment 2 (1-tert-butoxycarbonyl-3-[(S)-N-methyl-1-benzene containing 1mol ethylamino]-4-methyl-pyridine), heated to reflux until all the solids were dissolved, cooled to 0°C, crystallized, and recrystallized twice to obtain (3R,4R)-1-tert-butoxycarbonyl-3- [(S)-N-methyl-1-phenylethylamino]-4-methyl-pyridine-L-tartrate, the yield is 78.3% Structural formula:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com