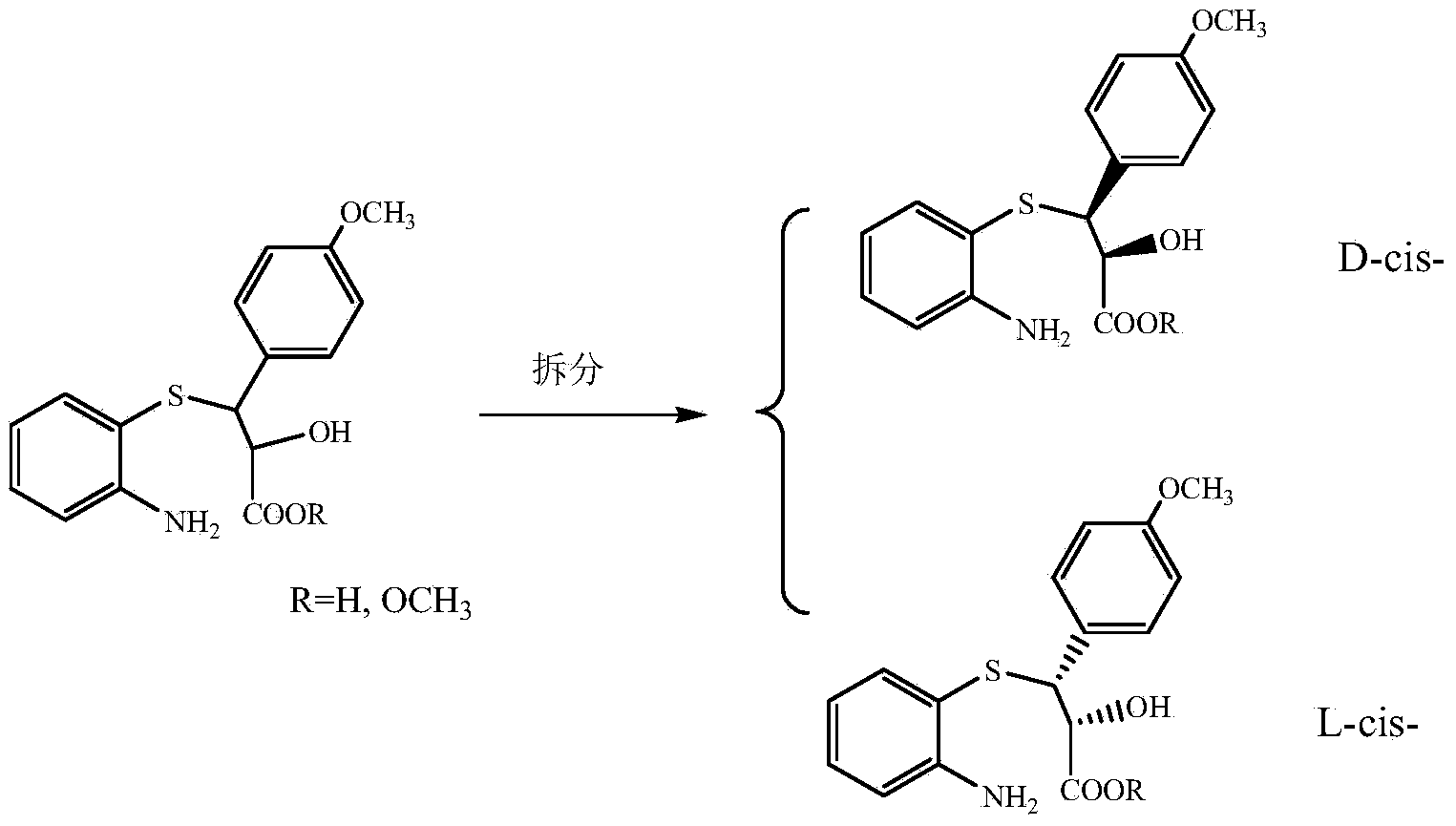
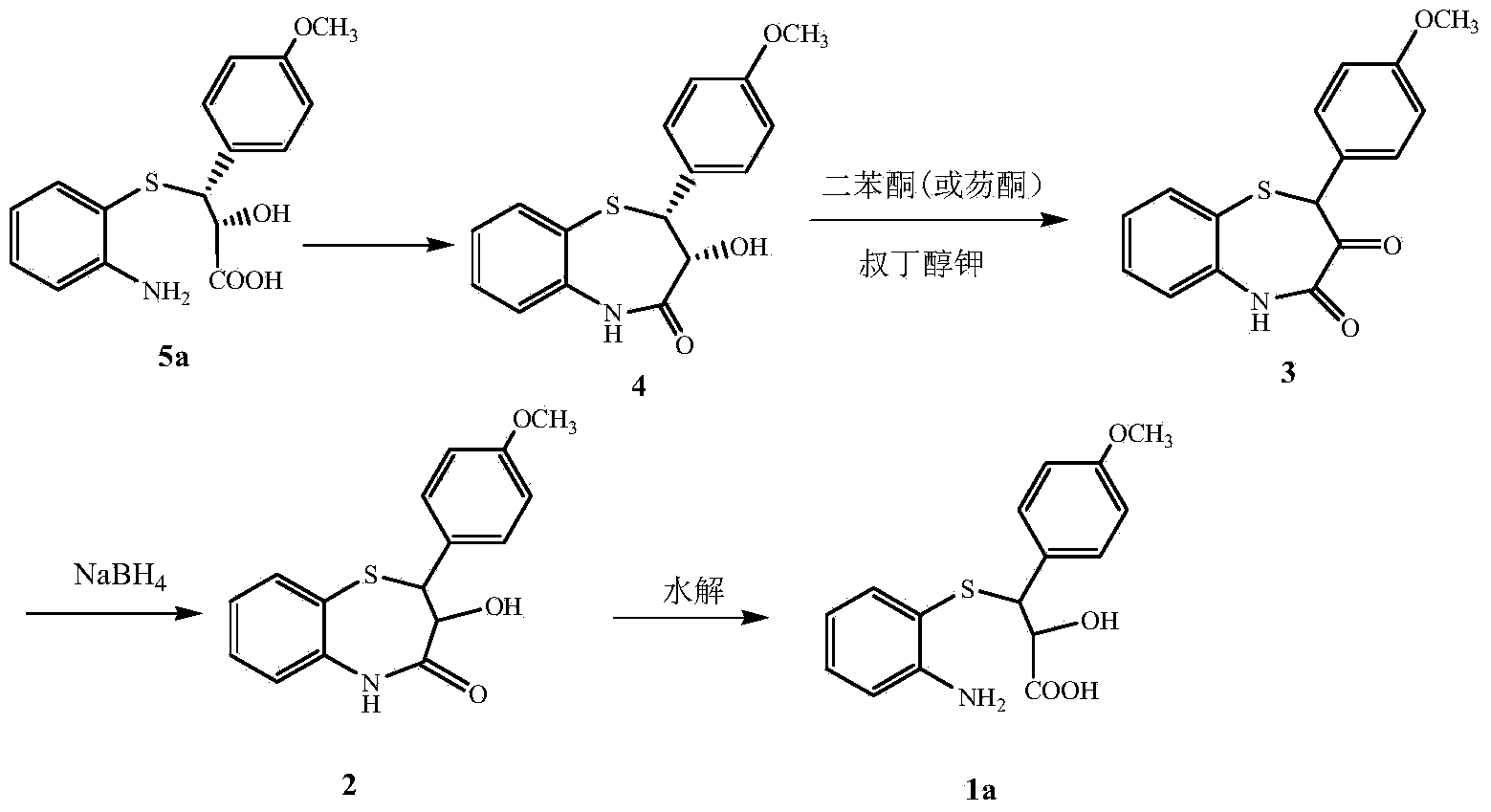
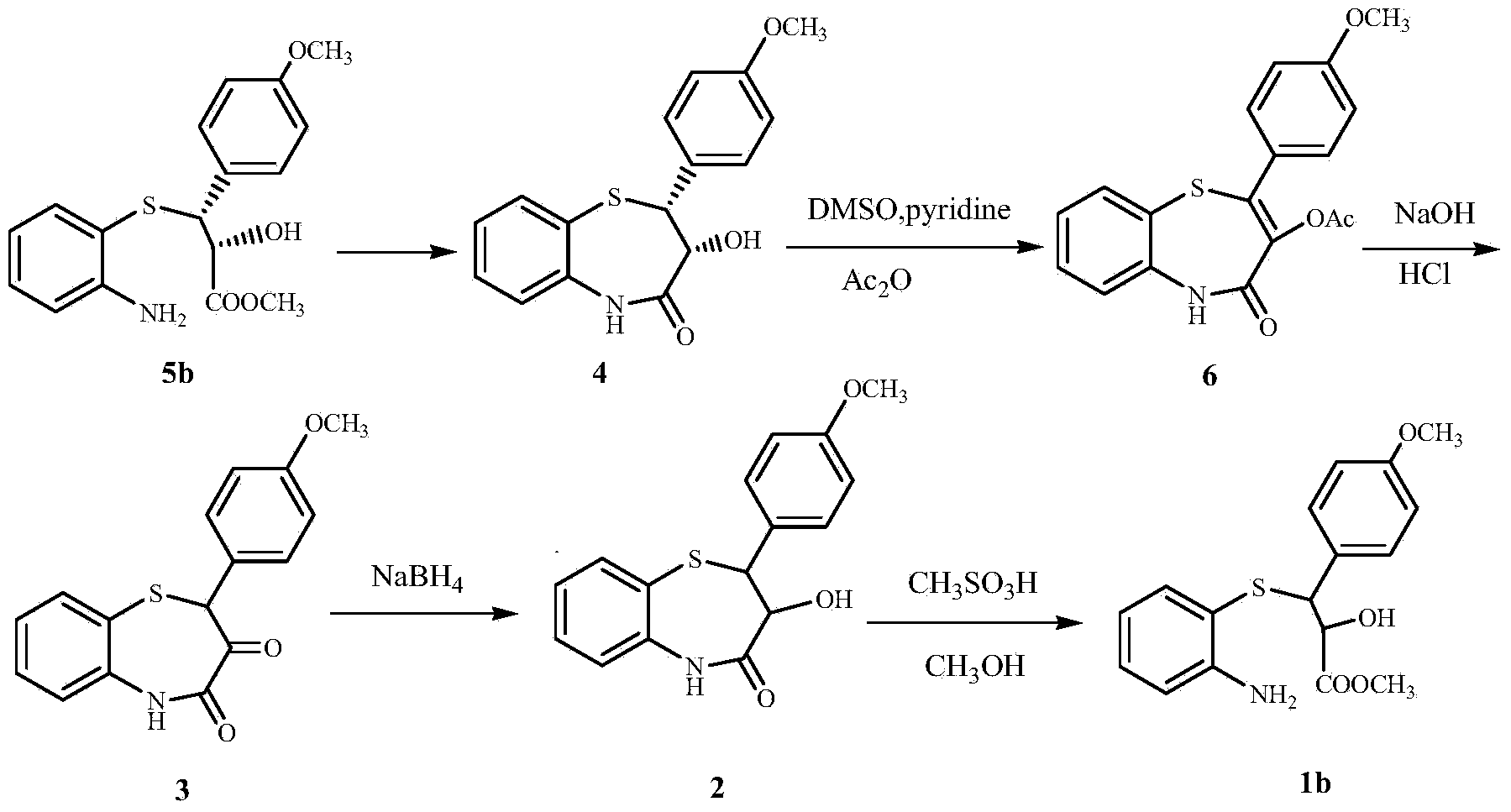
Process for recycling L-cis-lactam as diltiazem intermediate by-product
A technology of l-cis- and diltiazem, applied in organic chemistry and other directions, can solve problems such as route extension and long reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 5g (0.017mol) of L-cis-lactam (4) in 25ml of DMSO (containing 10% water), then add 5.35g (0.019mol) of IBX, and stir at 80°C for 6h. After the reaction is complete, pour into 200ml of cold water, stir for 1 hour, and then filter to collect the filter cake. The filter cake was washed with ethyl acetate, the mother liquor was washed with water, dried, and concentrated under reduced pressure to obtain 2-(4-methoxyphenyl)-1,5-benzothiazepine-3,4-(2H,5H)- Diketone (3), 4.5g, yield 90%, mp161-163°C, e.e.% is 0. The filter cake was dried and recovered to obtain IBA, 4.49g, with a recovery rate of 89%.
Embodiment 2
[0022] Dissolve 5g (0.017mol) of L-cis-lactam (4) in 25ml of DMSO (containing 5% water), then add 9.33g (0.034mol) of IBX, and stir at 5°C for 10h. After the reaction is complete, pour into 200ml of cold water, stir for 1 hour, and then filter to collect the filter cake. The filter cake was washed with ethyl acetate, the mother liquor was washed with water, dried, and concentrated under reduced pressure to obtain 2-(4-methoxyphenyl)-1,5-benzothiazepine-3,4-(2H,5H)- Diketone (3), 4.62g, yield 92%, mp162-163°C, e.e.% is 0. The filter cake was dried and recovered to obtain IBA, 7.75g, with a recovery rate of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com