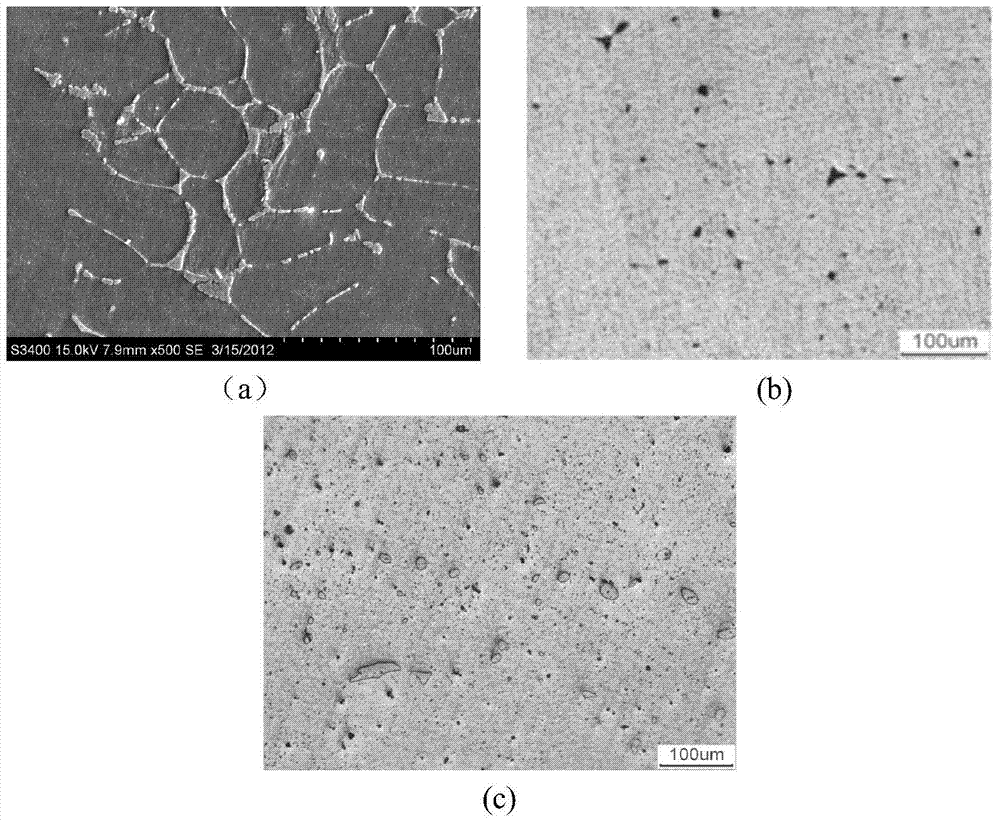
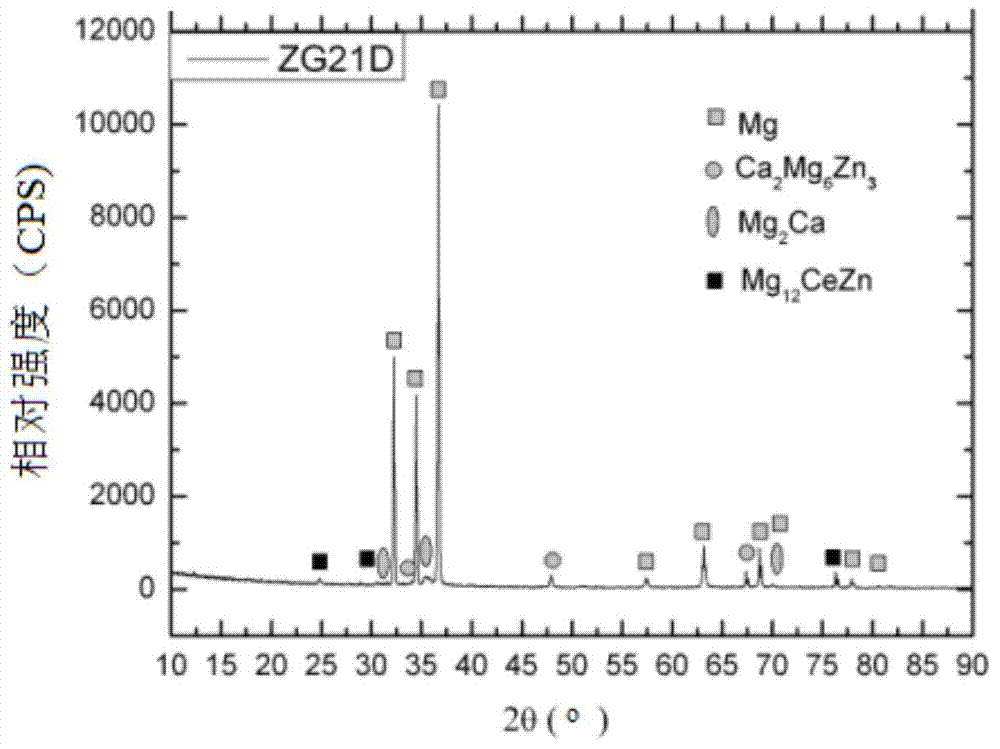
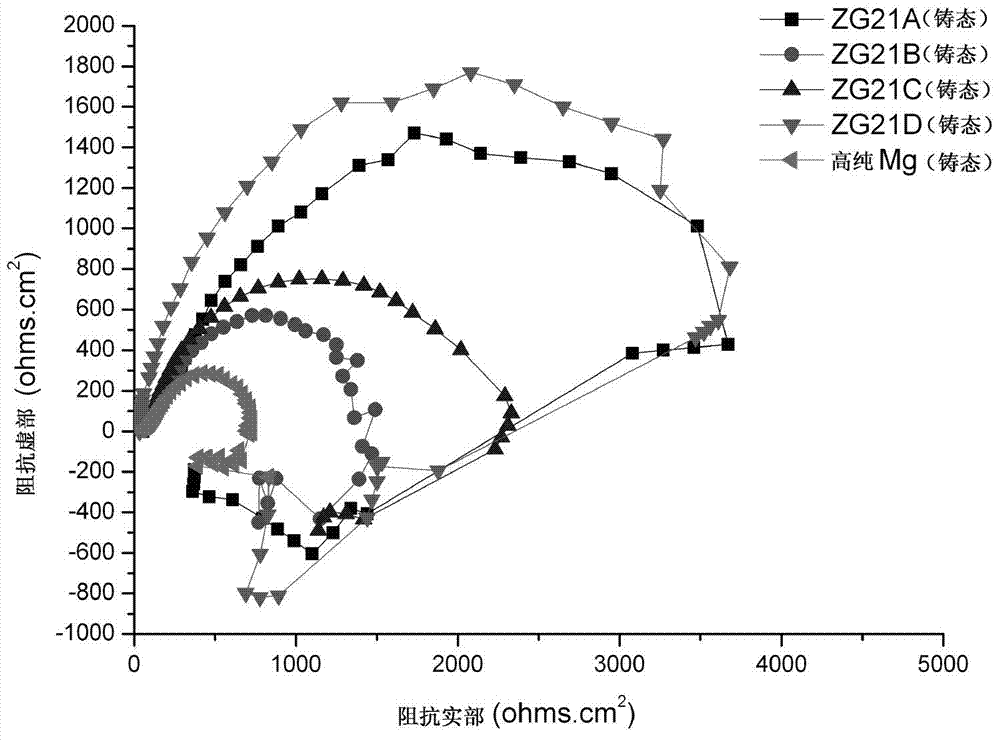
Dispersion strengthened medical Mg-Zn-Ce-Ca-Mn alloy and preparation method thereof
A technology of dispersion strengthening and alloying, applied in medical science, prosthesis, etc., can solve problems such as little progress, improve mechanical properties and corrosion resistance, simplify process operation and equipment requirements, improve processing performance and mechanical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. According to the design composition requirements of Mg-2.1Zn-1.4Ce-1.0Ca-0.5Mn medical magnesium alloy, high-purity magnesium (99.99%Mg), high-purity zinc (99.99%Zn), magnesium-cerium master alloy (containing 25%Ce ), magnesium-calcium master alloy (containing 32% Ca) and magnesium-manganese master alloy (containing 6% Mn) as raw materials, fully considering the element burning loss during the smelting process, the ratio of the addition amount of each raw material is calculated as 235:6:20 :10:30.
[0035]2. According to the composition design requirements of the new magnesium alloy described in 1, the melting and casting of the alloy and the solid solution and aging treatment of the cast slab are carried out, and the specific preparation steps are as follows:
[0036] 1) in SF 6 +CO 2 The Mg-Zn-Ce-Ca-Mn alloy ingot was smelted in a mixed protective atmosphere at a smelting temperature of 1033K, SF 6 +CO 2 The flow ratio of protective gas is 1:100 (flow rate SF ...
Embodiment 2
[0040] 1. According to the design composition requirements of Mg-2.0Zn-0.9Ce-0.9Ca-0.4Mn medical magnesium alloy, high-purity magnesium (99.99%Mg), high-purity zinc (99.99%Zn), magnesium-cerium master alloy (containing 25%Ce ), magnesium-calcium master alloy (containing 32% Ca) and magnesium-manganese master alloy (containing 6% Mn) as raw materials, fully considering the element burning loss during the smelting process, the ratio of the addition amount of each raw material is calculated as 245:6:14 :10:30.
[0041] 2. According to the composition design requirements of the new magnesium alloy described in 1, the melting and casting of the alloy and the solid solution and aging treatment of the cast slab are carried out, and the specific preparation steps are as follows:
[0042] 1) in SF 6 +CO 2 The Mg-Zn-Ce-Ca-Mn alloy ingot was smelted in a mixed protective atmosphere at a smelting temperature of 1033K, SF 6 +CO 2 The flow ratio of protective gas is 1:100 (flow rate SF ...
Embodiment 3
[0046] 1. According to the design composition requirements of Mg-1.9Zn-0.5Ce-0.9Ca-0.4Mn medical magnesium alloy, high-purity magnesium (99.99%Mg), high-purity zinc (99.99%Zn), magnesium-cerium master alloy (containing 25%Ce) ), magnesium-calcium master alloy (containing 32% Ca) and magnesium-manganese master alloy (containing 6% Mn) as raw materials, fully considering the element burning loss during the smelting process, the ratio of the addition amount of each raw material is calculated as 248:6:7 :10:30.
[0047] 2. According to the composition design requirements of the new magnesium alloy described in 1, the melting and casting of the alloy and the solid solution and aging treatment of the cast slab are carried out, and the specific preparation steps are as follows:
[0048] 1) in SF 6 +CO 2 The Mg-Zn-Ce-Ca-Mn alloy ingot was smelted in a mixed protective atmosphere at a smelting temperature of 1033K, SF 6 +CO 2 The flow ratio of protective gas is 1:100 (flow rate SF ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com