Hydrochloric acid circulation method for preparing ferrous chloride tetrahydrate, iron oxide red and sulfuric acid by using ferrous sulfate septihydrate
A technology of ferrous sulfate and ferrous chloride, which is applied in the field of clean technology, can solve the problem of unsolved by-product ammonium sulfate treatment, and achieve the effects of improving competitive advantage, great social benefits, and reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
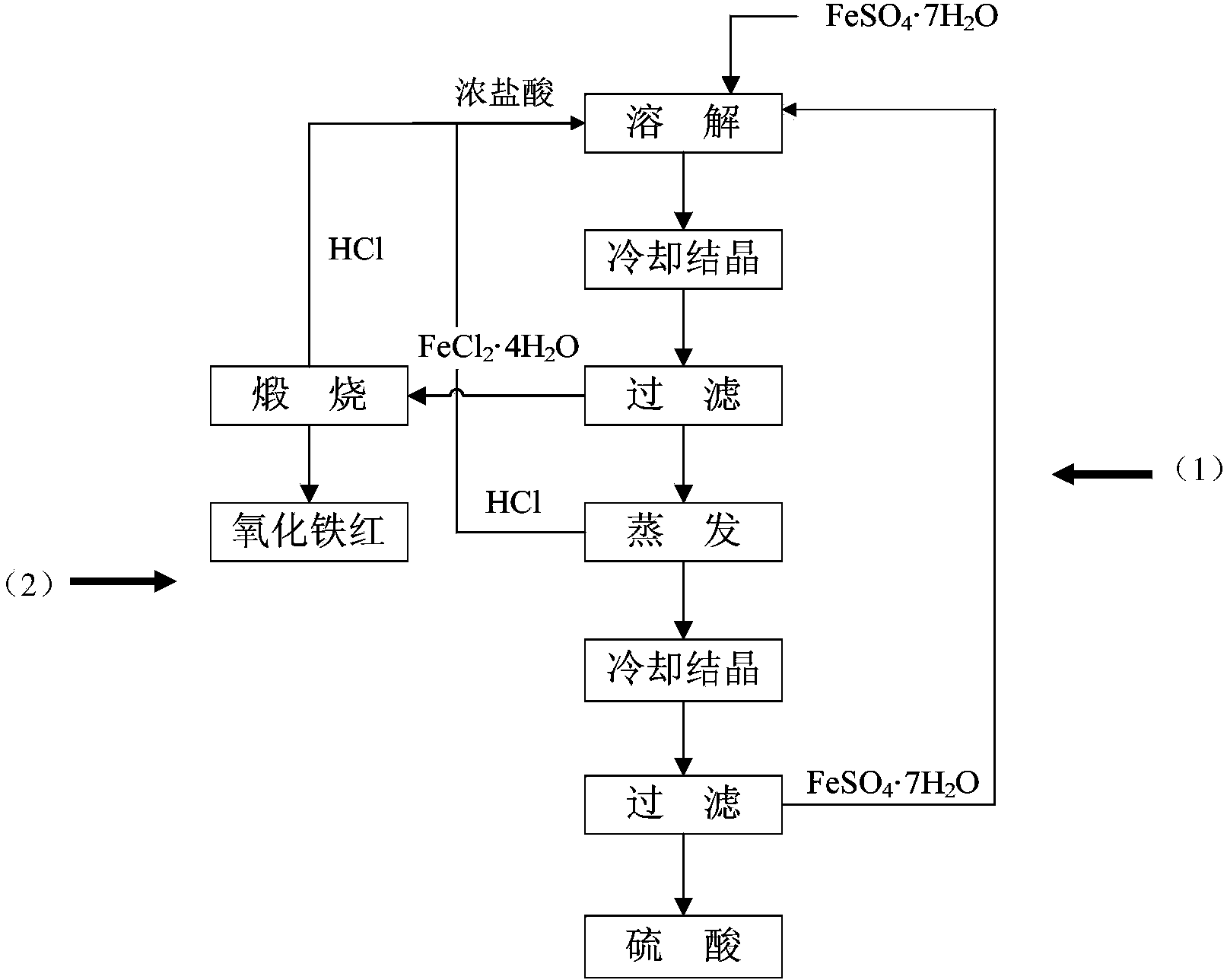
[0050] (1) Dissolve 400g of ferrous sulfate heptahydrate in 500ml of concentrated hydrochloric acid with a concentration of 37% at 70°C;
[0051] (2) Under the stirring condition of 300rpm, let the solution cool down slowly to 30°C, then ferrous chloride tetrahydrate will be precipitated, and the crystallization time will be maintained for 8 hours. After completion, ferrous chloride tetrahydrate can be obtained by filtering, washing and drying The product is about 100g;
[0052] (3) Calcining the obtained ferrous chloride tetrahydrate at 500°C for 1.5 hours to obtain about 40 g of iron oxide red, and return the HCl produced by calcination to step 1 for recycling;
[0053] (4) Evaporate the filtrate at 130°C to separate HCl, and freeze and crystallize at 10°C to separate about 250 g of ferrous sulfate heptahydrate to obtain sulfuric acid with a concentration of 60%. The separated HCl and ferrous sulfate heptahydrate are returned to step 1 for recycling.
Embodiment 2
[0055] (1) Dissolve 300g of ferrous sulfate heptahydrate in 500ml of concentrated hydrochloric acid with a concentration of 37% at 80°C;
[0056] (2) Under the stirring condition of 300rpm, let the solution cool down slowly to 20°C, then ferrous chloride tetrahydrate will be precipitated, and the crystallization time will be kept for 6 hours. After completion, ferrous chloride tetrahydrate can be obtained by filtering, washing and drying The product is about 80g;
[0057] (3) Calcining the obtained ferrous chloride tetrahydrate at 600°C for 1 hour to obtain about 30 g of iron oxide red, and return the HCl produced by calcination to step 1 for recycling;
[0058] (4) The filtrate was evaporated at 130°C to separate HCl, and 180 g of ferrous sulfate heptahydrate was separated by freezing and crystallization at 10°C to obtain sulfuric acid with a concentration of 65%. The separated HCl and ferrous sulfate heptahydrate were returned to step 1 for recycling.
Embodiment 3
[0060] (1) Dissolve 400g of ferrous sulfate heptahydrate in 500ml of 35% concentrated hydrochloric acid at 80°C;
[0061] (2) Under the stirring condition of 400rpm, let the solution cool down slowly to 20°C, then ferrous chloride tetrahydrate will be precipitated, and the crystallization time will be kept for 10h. After completion, ferrous chloride tetrahydrate can be obtained by filtering, washing and drying The product is about 90g;
[0062] (3) Calcining the obtained ferrous chloride tetrahydrate at 500°C for 0.5 hours to obtain about 35 g of iron oxide red, and return the HCl produced by calcination to step 1 for recycling;
[0063] (4) Evaporate and separate HCl from the filtrate at 130°C, and separate 260 g of ferrous sulfate heptahydrate by freezing and crystallizing at 10°C to obtain sulfuric acid with a concentration of 63%. The separated HCl and ferrous sulfate heptahydrate are returned to step 1 for recycling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com