A kind of preparation method of rifampicin Ⅱ crystal form
A technology of rifampicin and crystal form, which is applied in the crystallization field of chemical engineering industry, can solve the problems of long cooling crystallization time, poor working environment, and small product particle size, and achieve the effect of large crystal particle size, uniform distribution, and increased bulk density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
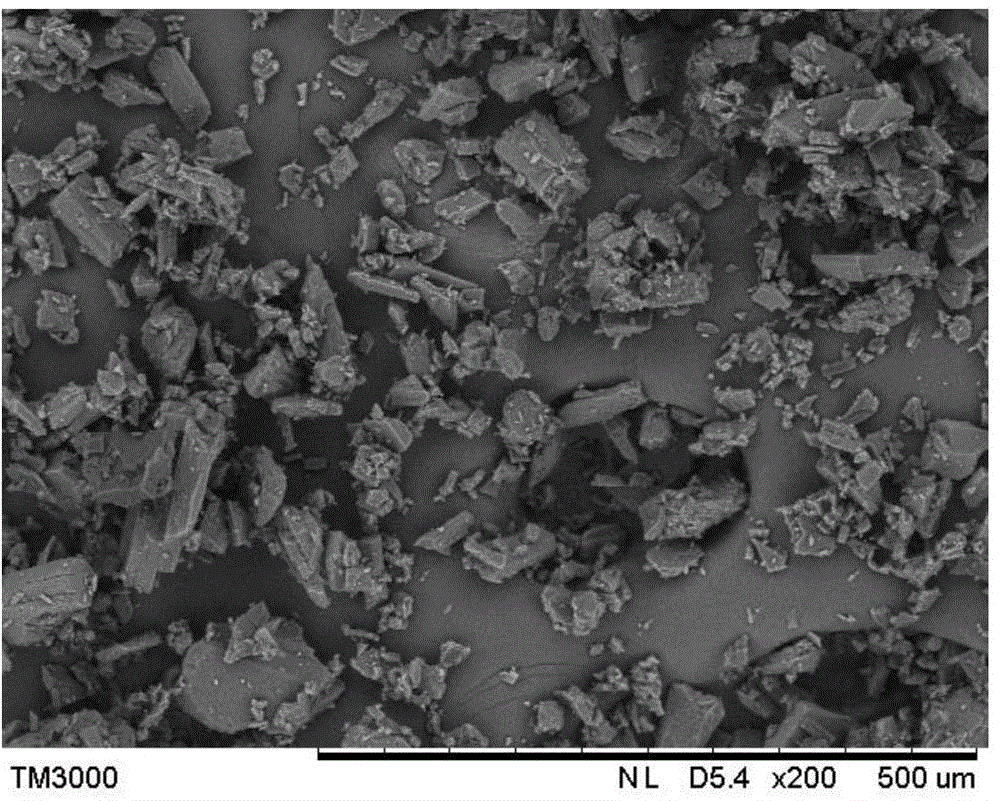
[0034]Dissolve 160 g of Form I rifampicin with a purity of 90% in 1000 mL of acetone under stirring, add 25 mL of water, and heat up to 57° C. to completely dissolve the rifampicin solid; then start vacuum evaporation with a vacuum of 0.01 MPa, and the solution The evaporation temperature is 55°C, and the evaporation rate is 200mL / hr. When the volume of the distillate reaches 500mL, the evaporation is stopped; the temperature is lowered to 10°C at a cooling rate of 4°C / h. The crystalline slurry was filtered, washed, and dried at 35°C for 3 hours to obtain 124.8 g of the crystal form II rifampicin product, with a yield of 78%.
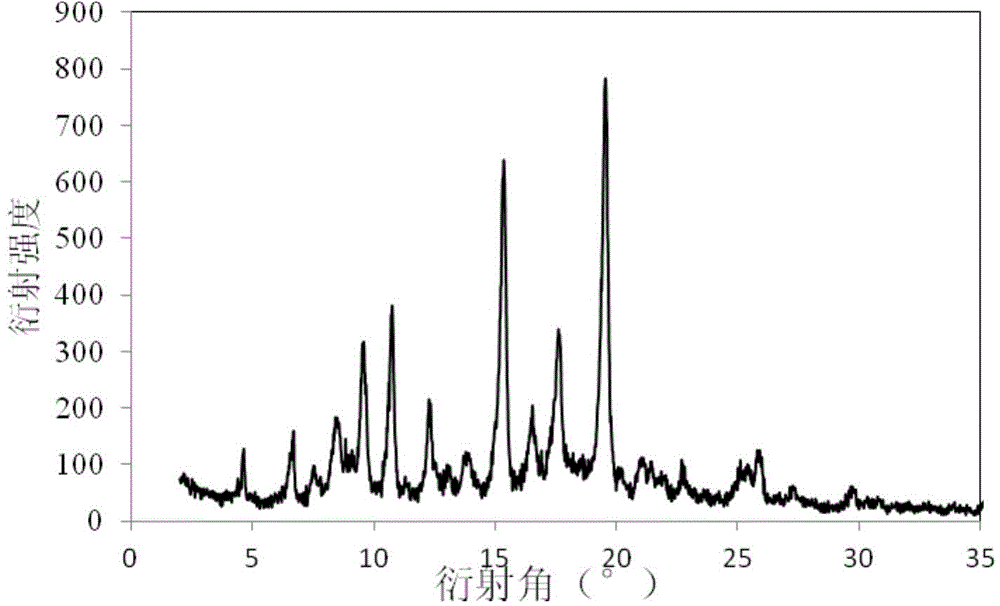
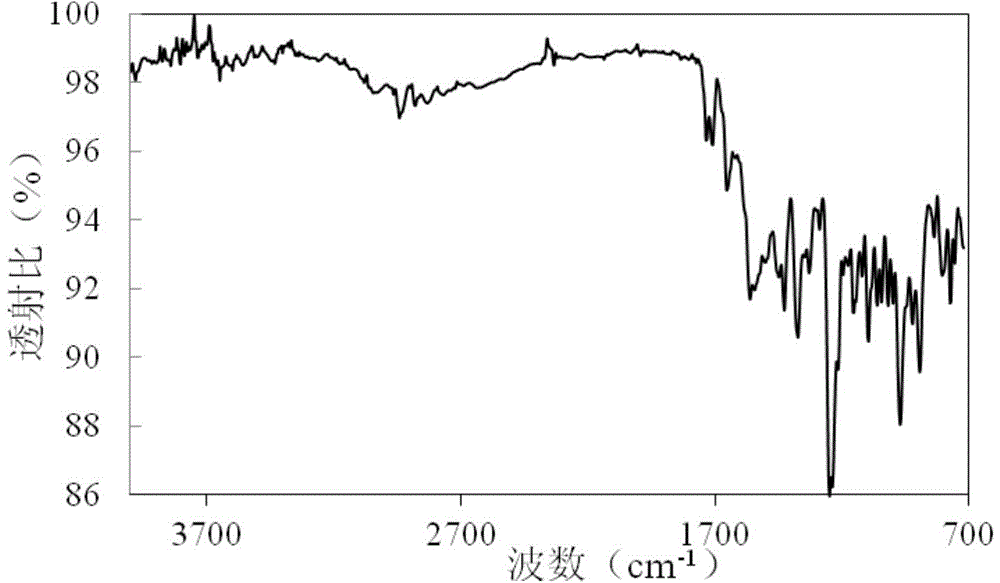
[0035] The powder X-ray diffraction pattern of the product is as figure 2 As shown, there are characteristic peaks at diffraction angles 2θ of 4.62, 6.73, 7.48, 8.42, 9.47, 10.70, 12.21, 13.08, 13.94, 15.33, 16.51, 17.58, 19.57, 21.06, 22.73, 25.14, 25.92, 27.28 and 29.69 degrees . IR image 3 Shown at 773, 805, 838, 894, 971, 1000, 1019, 1044, 1096...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com