A novel sulfhydryl fluorescent probe, its preparation method and its application
A fluorescent probe and sulfhydryl technology, applied in the field of preparation and new sulfhydryl fluorescent probes, can solve the problems of lack of selectivity, fixed-point labeling of proteins, and inability to respond well to the biological properties of polysaccharides, and achieve high fluorescence intensity and stability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
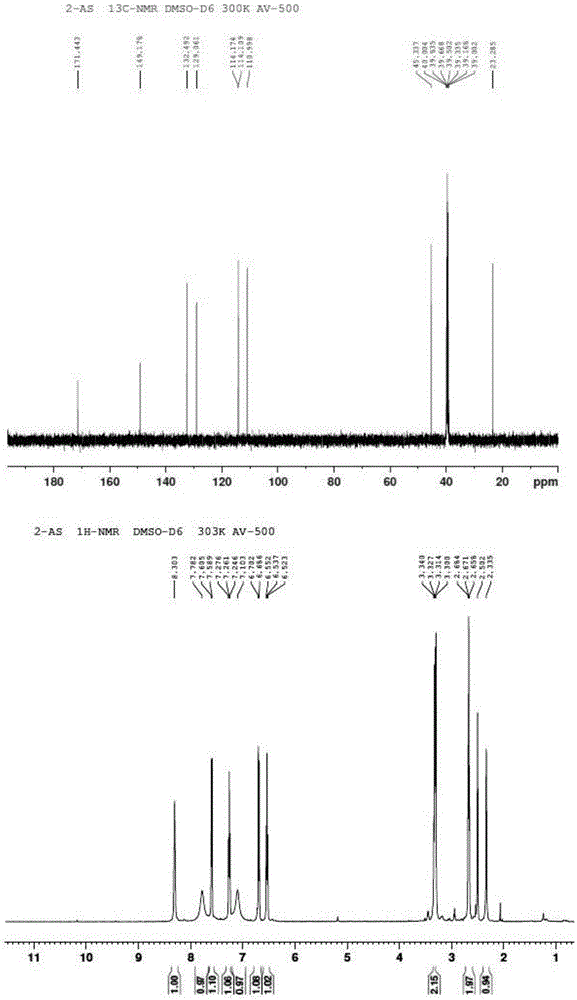
[0046] The preparation of embodiment 12-(2-mercaptoethylamino) benzamide
[0047] 1) Dissolve 19ml of chloroacetaldehyde solution with a mass percentage of 40% in 40ml of aqueous solution, cool to 0°C in an ice bath to obtain diluted chloroacetaldehyde solution, add dropwise a sodium carbonate solution with a concentration of 2Mol / L, and adjust the pH to 3 ;
[0048] 2) Dissolve 9.9g of sodium hydrosulfide in 33ml of water to obtain an aqueous solution of sodium hydrosulfide, cool it to 0°C in an ice bath, and add it dropwise to the chloroacetaldehyde solution at a rate of 2-3 drops per second, and at the same time, at 200 rpm Stir the chloroacetaldehyde solution at a constant speed, and a white solid precipitates out in the solution. After the sodium hydrosulfide is added, filter it with suction and dry it in vacuum to obtain mercaptoaldehyde;
[0049] 3) Dissolve 434mg of mercaptoaldehyde in 120ml of methanol solution, stir until dissolved at room temperature, add 1.5g of 2-a...
Embodiment 2
[0052] The preparation of embodiment 22-(2-mercaptoethylamino) benzoylformic acid
[0053] 1) 28.5ml mass percentage is that 40% chloroacetaldehyde solution is dissolved in 60ml aqueous solution, is cooled to 0 ℃ in ice bath, obtains the diluted chloroacetaldehyde solution, drips the sodium carbonate solution that concentration is 2Mol / L, adjusts pH to be 3 or so;
[0054] 2) Dissolve 15g of sodium hydrosulfide in 50ml of water to obtain an aqueous solution of sodium hydrosulfide, cool it to 0°C in an ice bath, and add it dropwise to the chloroacetaldehyde solution at a rate of 2-3 drops per second, at the same time at a rate of 200 rpm Constantly stirring the chloroacetaldehyde solution, a white solid precipitates out in the solution, and after the sodium hydrosulfide is added, it is suction filtered and vacuum-dried to obtain mercaptoaldehyde;
[0055] 3) Dissolve 108 mg of mercaptoaldehyde in 50 ml of methanol solution, stir at room temperature until dissolved, add 304 mg ...
Embodiment 3
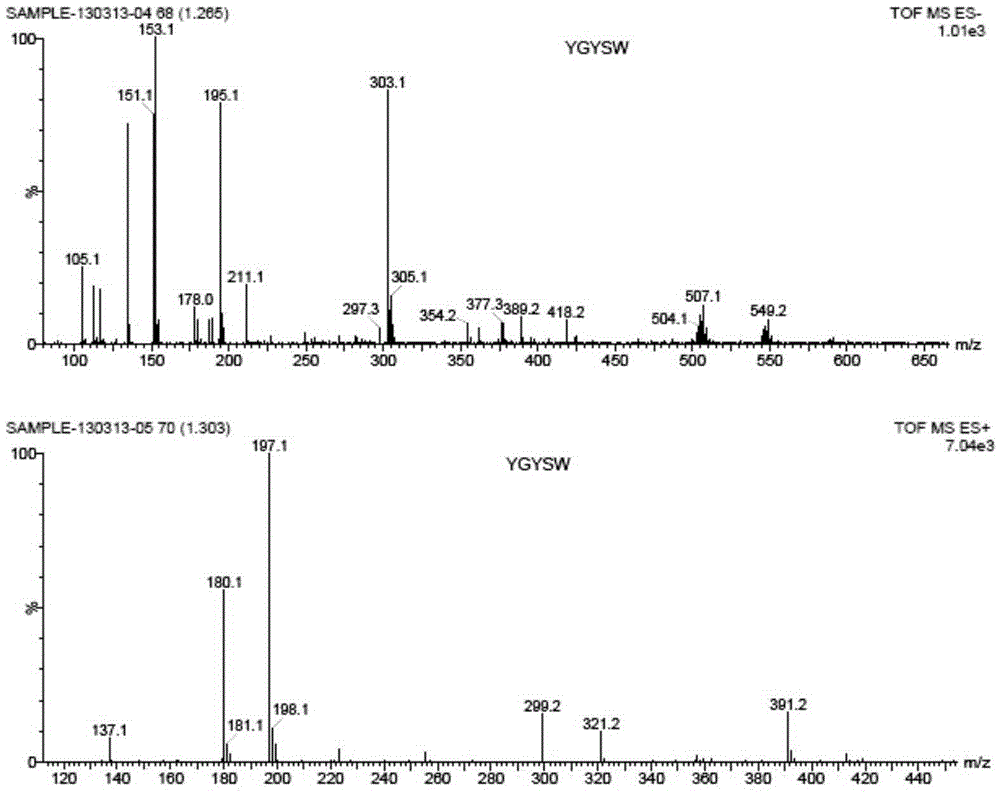
[0058] Example 3 Using the compound of the present invention to specifically label the non-reducing end of heparin
[0059] 1. Use 2-(2-mercaptoethylamino)benzamide to specifically label the non-reducing end of heparin
[0060] Add 500mg of enoxaparin into 3.6ml of water, stir to dissolve, and add 1.27mg of benzethonium chloride into 1.1ml of water, slowly drop the benzethonium chloride solution into the low molecular weight heparin sodium solution, and stir for 1h , washed with water 3 times, filtered, and dried under reduced pressure to obtain 240 mg of benzethon chloramine salt.
[0061] Take 100mg of benzethon chloramine salt and add 2ml of dichloromethane, stir to dissolve, add 110ul benzyl chloride, stir at room temperature for 24h, add sodium acetate-methanol solution after the reaction, stir for 30min, remove the supernatant, wash with methanol for 3 times, filtered, dried under reduced pressure, and freeze-dried to obtain low molecular weight heparin benzyl ester.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com