Cartilage cell treating agent comprising collagen, hyaluronic acid derivative, and stem cell derived from mammal umbilical cord
A chondrocyte and hyaluronic acid technology, applied in the field of chondrocyte therapeutics, can solve problems such as cell leakage, preventing tissue regeneration, and inability to adequately treat diseased areas.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
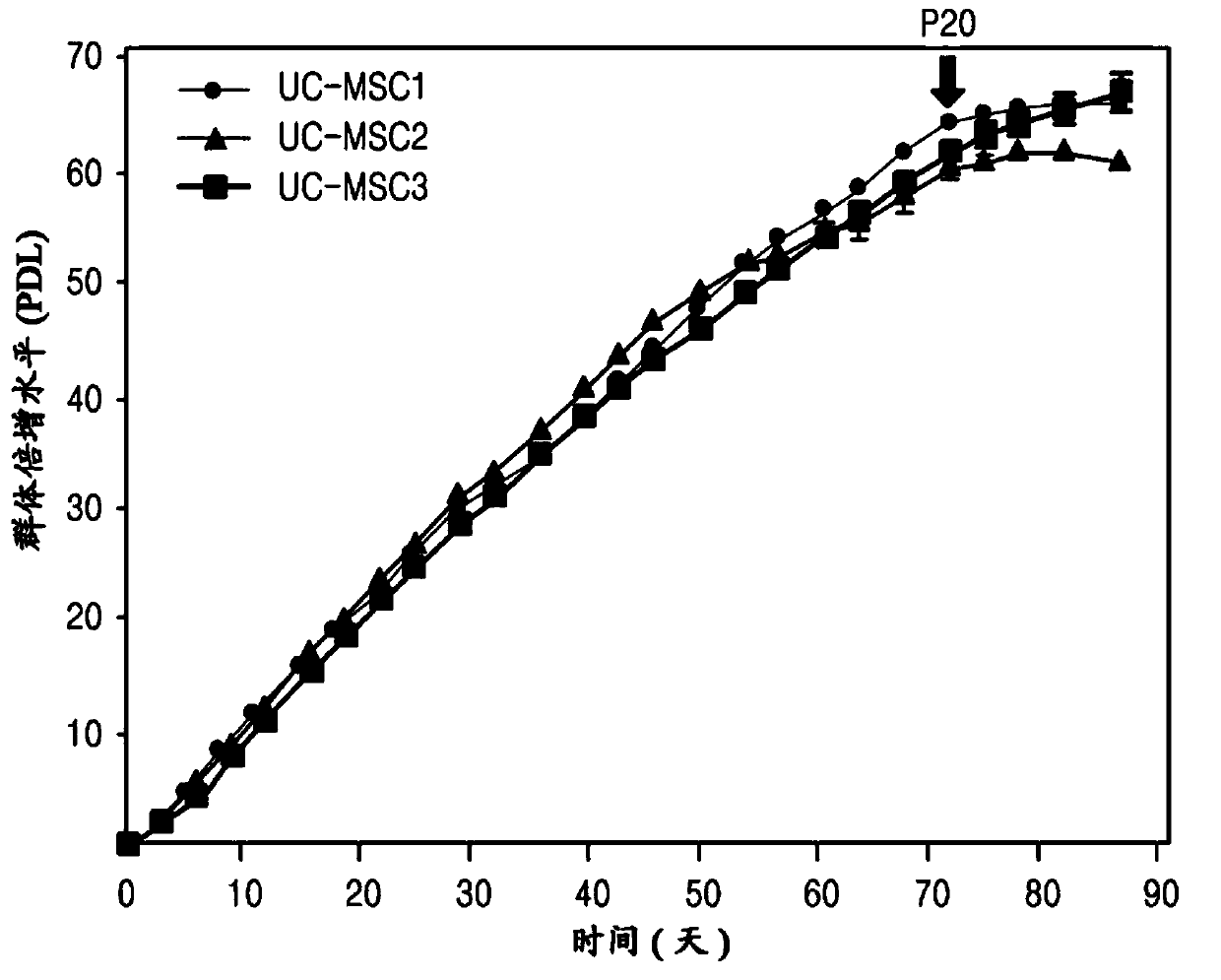
[0072] Analysis of Separation and Proliferation Efficiency of Umbilical Cord Stem Cells
[0073] The umbilical cords used in this study were discarded after delivery and obtained with the consent of normal mothers. Use the umbilical cord within 24 hours of collecting it.
[0074] Tissues from the blood outside the umbilical cord were removed by using Ca-free 2+ and Mg 2+ DPBS to remove the outer amniotic membrane and two arteries, cut to 1mm 3 size, and then placed in α-minimal essential medium (α-MEM) containing 100 U / mL penicillin, 0.1 μg / mL streptomycin and 0.2 μg / mL umbilical cord extract. After culturing them for 7 days, when the cells appeared attached to the bottom, the cells were treated with α-MEM containing 500 U / ml type I collagenase for 4 hours to detach the cells. Afterwards, the cells were divided into 2 × 10 3 piece / cm 2 Inoculate the culture dish (containing α-MEM, the dish contains 100U / ml penicillin, 0.1μg / ml streptomycin and 0.2mg / ml umbilical cord ex...
Embodiment 2
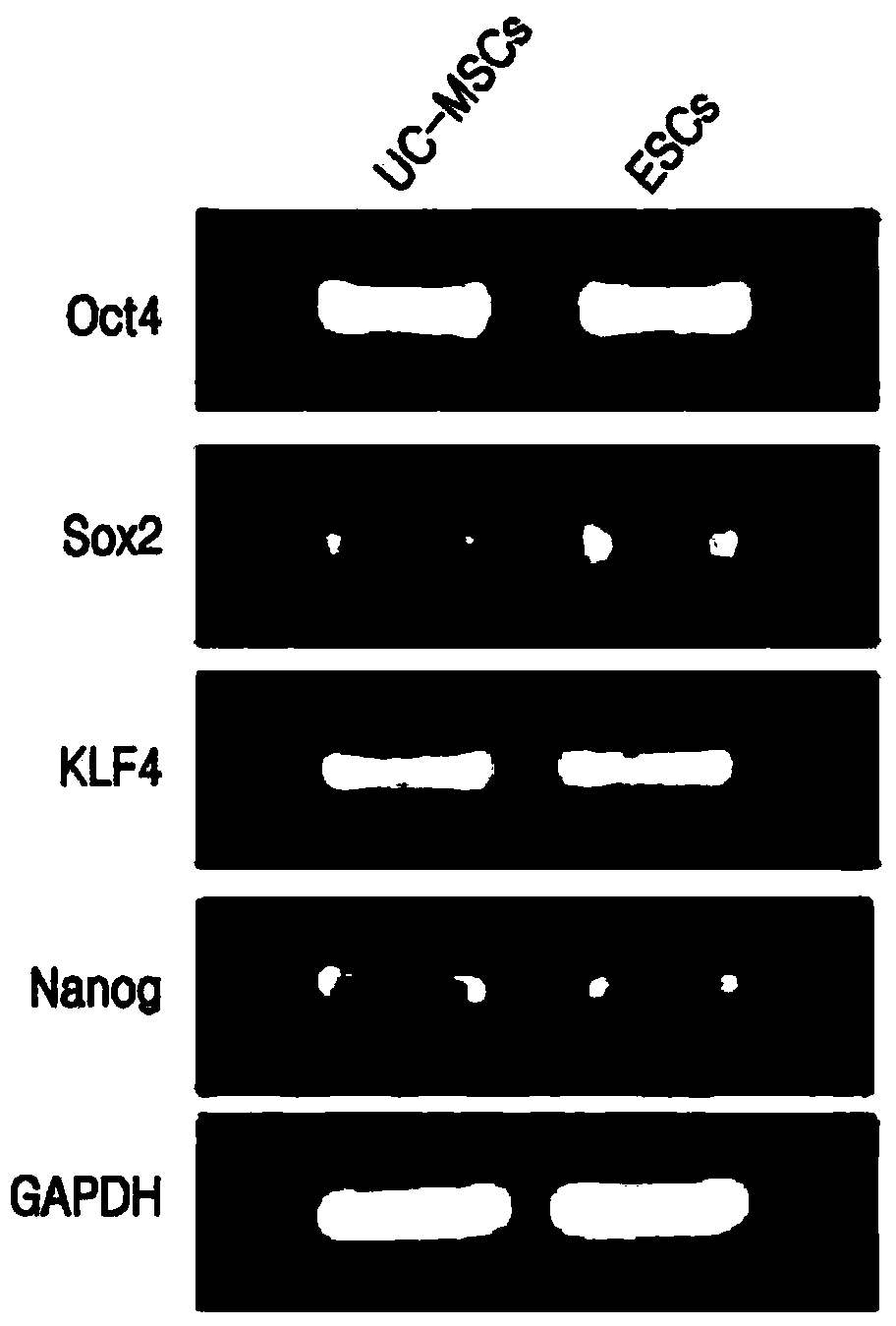
[0075] Analysis of embryonic stem cell markers by RT-PCR
[0076] Cell pellets were treated with Ca-free 2+ and Mg 2+ was washed with DPBS, 1 ml of lysis buffer (iNtRON Biotechnology product) was added thereto, and then total RNA was isolated therefrom according to the method described in the manual available from iNtRON Biotechnology. 1 μg of RNA was reverse-transcribed in 20 μL of reaction solution containing reaction buffer, 1 mM dNTP mixture, 0.5 μg / μL oligo(dT)15, 20 U RNase inhibitor and 20U of AMV reverse transcriptase. The reaction was carried out at a temperature of 42°C for 60 minutes. The RT product (cDNA) obtained therefrom was subjected to PCR using a 2X PCR Master mixed solution kit (product of iNtRON Biotechnology), which contained 10 μL of a reaction solution containing 1X Taq buffer, 0.25 U Taq polymerase, 10 pM Sense and antisense gene-specific primers. Amplification was performed for a total of 32 cycles and each cycle included denaturation at 94°C for...
Embodiment 3
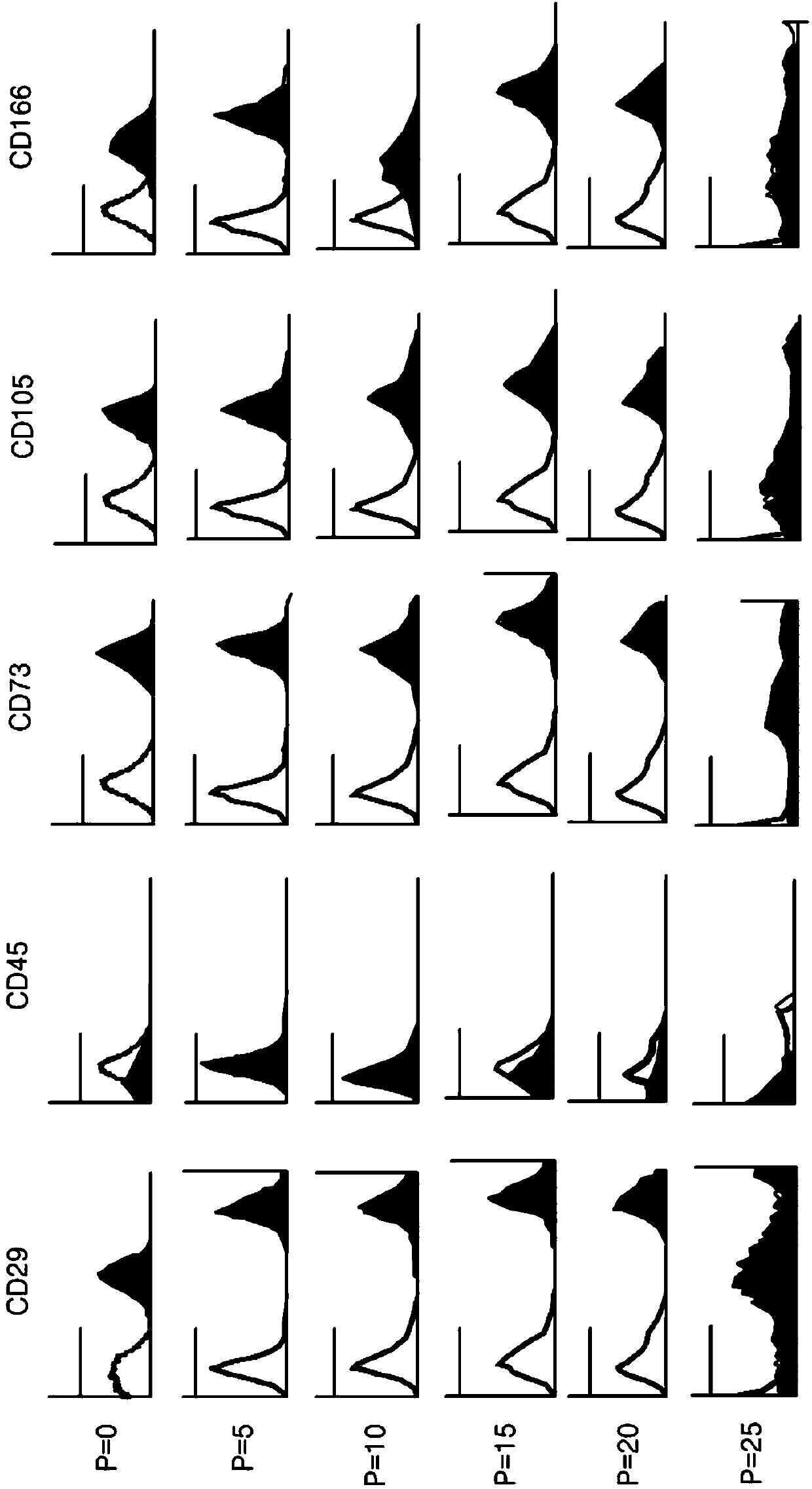
[0081] Analysis of the expression of mesenchymal stem cell markers by FACS
[0082] Flow cytometry was used to analyze the characteristics of the isolated cells. The isolated cells were washed with PBS, treated with trypsin-EDTA to prepare single-cell groups, and then washed with PBS containing 2% FBS and 1 mM EDTA. Thereafter, stem cell markers bound to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were treated and allowed to react for 20 minutes, and then analyzed with FACSCalibur (product of Becton-Dickinson).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com