Method for preparing high-purity 7-chloro-5-phenyl-benzodiazepine-2-one
A benzodiazepine, high-purity technology, applied in the field of pharmaceutical synthesis technology, can solve the problems of inability to separate out materials, low HPLC purity of intermediates, blockage of condensers, etc., to avoid blockage of condensers, avoid material discharge difficulties, The effect of avoiding material loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
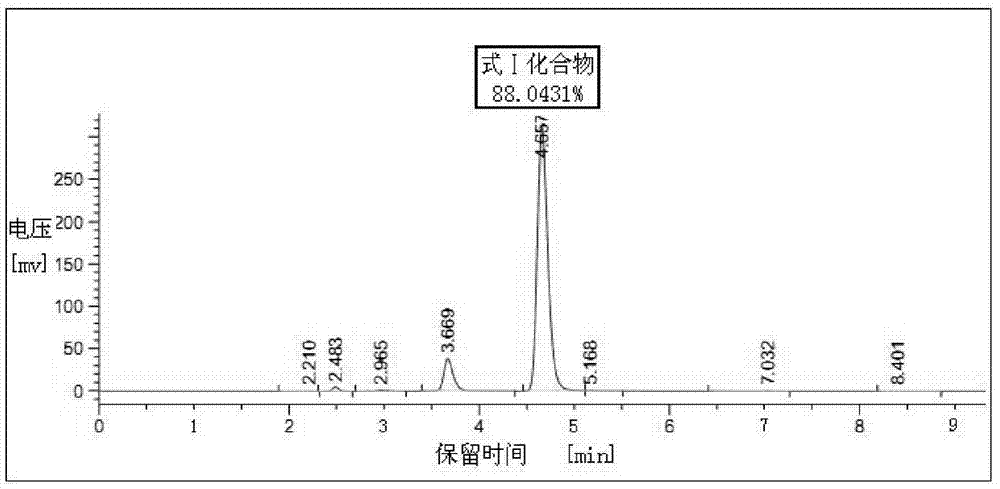
[0032]Put 400ml of industrial ethanol, 40g (0.130mol) of 2-chloroacetamido-5-chloro-benzophenone, and 34g of hexamethylenetetramine into the reaction bottle in turn. After stirring evenly, add dropwise at room temperature with a concentration of more than 35% by mass 15ml of refined hydrochloric acid. After the addition, the temperature was raised to reflux for 7 hours. Concentrate under reduced pressure to nearly dryness, cool down to room temperature, add 600ml of chloroform, stir to dissolve. Wash with 100ml×3 purified water, remove the water layer, and add 48ml (0.144mol) of 3mol / L dilute nitric acid dropwise to the chloroform solution under temperature control below 20°C, and gradually precipitate yellow solid particles. After the addition is complete, continue to stir and react at 20°C to 25°C for 2 hours. Filter, put the filter cake into 400ml of purified water and stir for 1 hour, cool down to below 10°C and add a saturated aqueous solution of sodium bicarbonate drop...
Embodiment 2
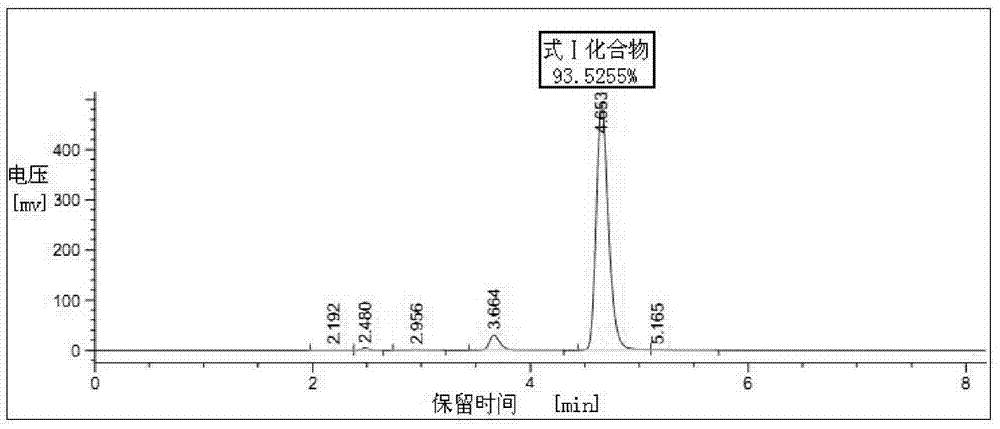
[0034] Put 300ml of industrial ethanol, 30g (0.097mol) of 2-chloroacetamido-5-chloro-benzophenone, and 25.5g of hexamethylenetetramine into the reaction bottle in turn. After stirring evenly, add dropwise at room temperature with a mass percentage concentration of 35% The above refined hydrochloric acid 12ml. After the addition, the temperature was raised to reflux for 7 hours. Concentrate under reduced pressure to nearly dryness, cool down to room temperature, add 600ml of chloroform, stir to dissolve. Wash with 75ml×3 purified water, remove the water layer, and then add 42ml (0.126mol) of 3mol / L dilute nitric acid dropwise to the chloroform solution under temperature control below 20°C, and gradually precipitate yellow solid particles. After the addition is complete, continue to stir and react at 20°C to 25°C for 2 hours. Filter, put the filter cake into 300ml of purified water and stir for 1 hour, cool down to below 10°C and add a saturated aqueous solution of sodium bica...
Embodiment 3
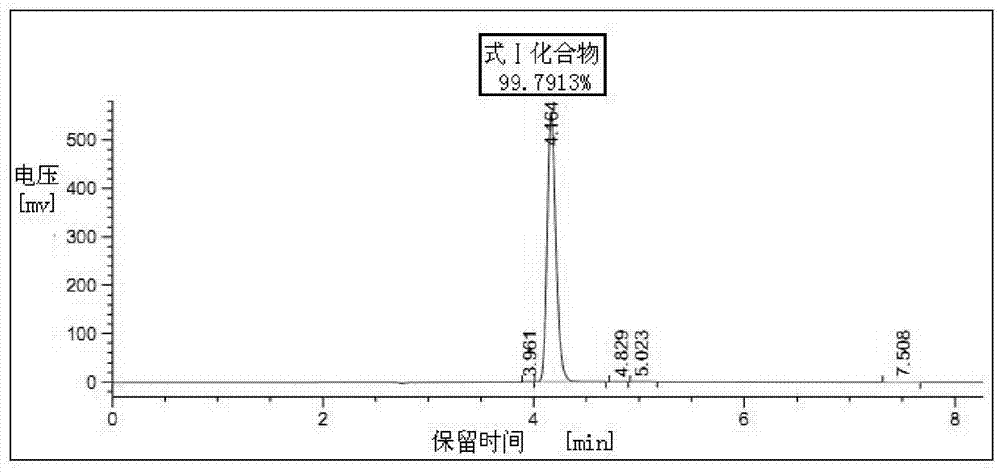
[0036] Put 500ml of industrial ethanol, 50g (0.162mol) of 2-chloroacetamido-5-chloro-benzophenone, and 42.5g of hexamethylenetetramine into the reaction bottle in turn. After stirring evenly, add dropwise at room temperature with a mass percentage concentration of 35% The above refined hydrochloric acid 19ml. After the addition, the temperature was raised to reflux for 7 hours. Concentrate under reduced pressure to nearly dryness, cool down to room temperature, add 875ml of chloroform, stir to dissolve. Wash with 125ml×3 purified water, remove the water layer, and then add 65ml (0.195mol) of 3mol / L dilute nitric acid dropwise to the chloroform solution under temperature control below 20°C, and gradually precipitate yellow solid particles. After the addition is complete, continue to stir and react at 20°C to 25°C for 2 hours. Filter, put the filter cake into 500ml of purified water and stir for 1 hour, cool down to below 10°C and add a saturated aqueous solution of sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com