Jevtana solvate and preparation method and application thereof
A technology of cabazitaxel and solvate, applied in the field of drug synthesis, can solve the problems of no reported solvate preparation method, poor stability of cabazitaxel anhydrous, uncharacterized solvate, etc., and achieves strong practical value and good stability. , the effect of weak hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
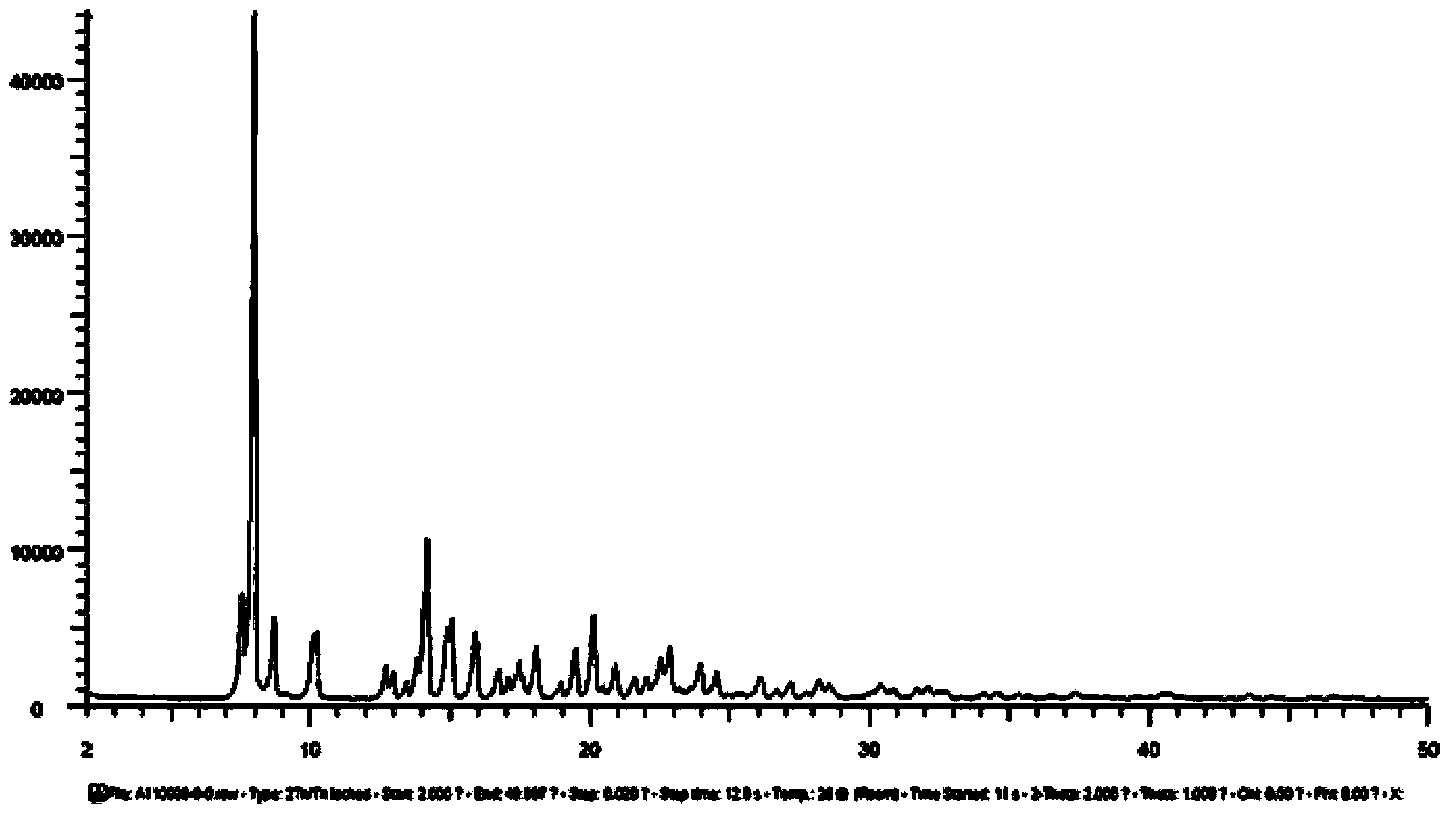
[0078] Add 25.0g of cabazitaxel raw material (HPLC purity 99.1%) into 250mL of ethyl acetate, heat to 70°C, stir to dissolve completely; naturally cool to 40°C, keep warm and continue to stir for 8 hours to precipitate crystals; then cool down naturally to 25° C., kept stirring for 2 hours; filtered, the filter cake was washed with 20 mL of ethyl acetate, and vacuum-dried at 40° C. for 8 hours to obtain 25.4 g of cabazitaxel ethyl acetate, which contained 9.47% ethyl acetate (theoretical amount was 9.53%), and the HPLC purity was 99.6%.
[0079] The HNMR data are as follows: 1 H NMR (400MHz, CDCl 3 )δ8.11(d,J=7.4Hz,2H),7.62(t,J=7.4Hz,1H),7.51(t,J=7.7Hz,2H),7.45–7.38(m,4H),7.37– 7.31(m,1H),6.23(t,J=8.3Hz,1H),5.65(d,J=6.9Hz,1H),5.47(d,J=9.6Hz,1H),5.30(d,J=9.21 H),4.99(d,J=8.1Hz,1H),4.81(s,1H),4.64(s,1H),4.32(d,J=8.5Hz,1H),4.16(dt,J=14.3,7.8 Hz,3H),3.87(dd,J=10.7,6.4Hz,1H),3.83(d,J=7.0Hz,1H),3.47(s,4H),3.32(s,3H),2.77-2.66(m ,1H),2.38(s,3H),2.30(d,J=9.2Hz,2H),2.19(s,1H),2.06...
Embodiment 2
[0091] Add 10.0 g of cabazitaxel raw material (HPLC purity 98.5%) into 200 mL of ethyl acetate, heat to 60 ° C, stir to dissolve completely; naturally cool to 40 ° C, add 0.05 g of cabazitaxel ethyl acetate solvate as crystal and keep stirring for 5 hours to precipitate crystals; then naturally cool down to 20°C, keep stirring for 2 hours; filter, wash the filter cake with 20mL of ethyl acetate, and dry in vacuum at 40°C for 8 hours to obtain 10.3g of cabazitaxel Ethyl acetate, which contains 9.25% ethyl acetate, HPLC purity is 99.5%.
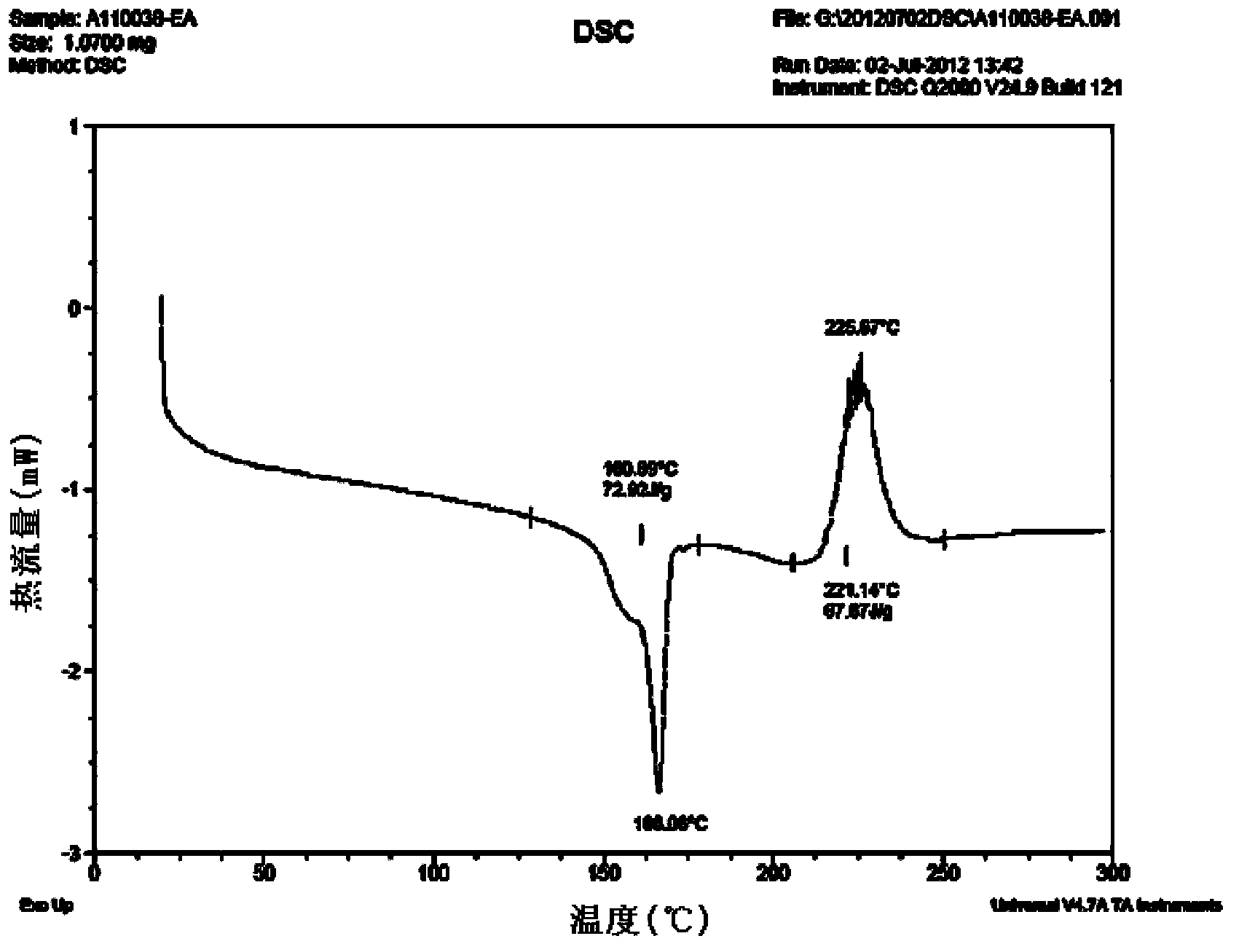
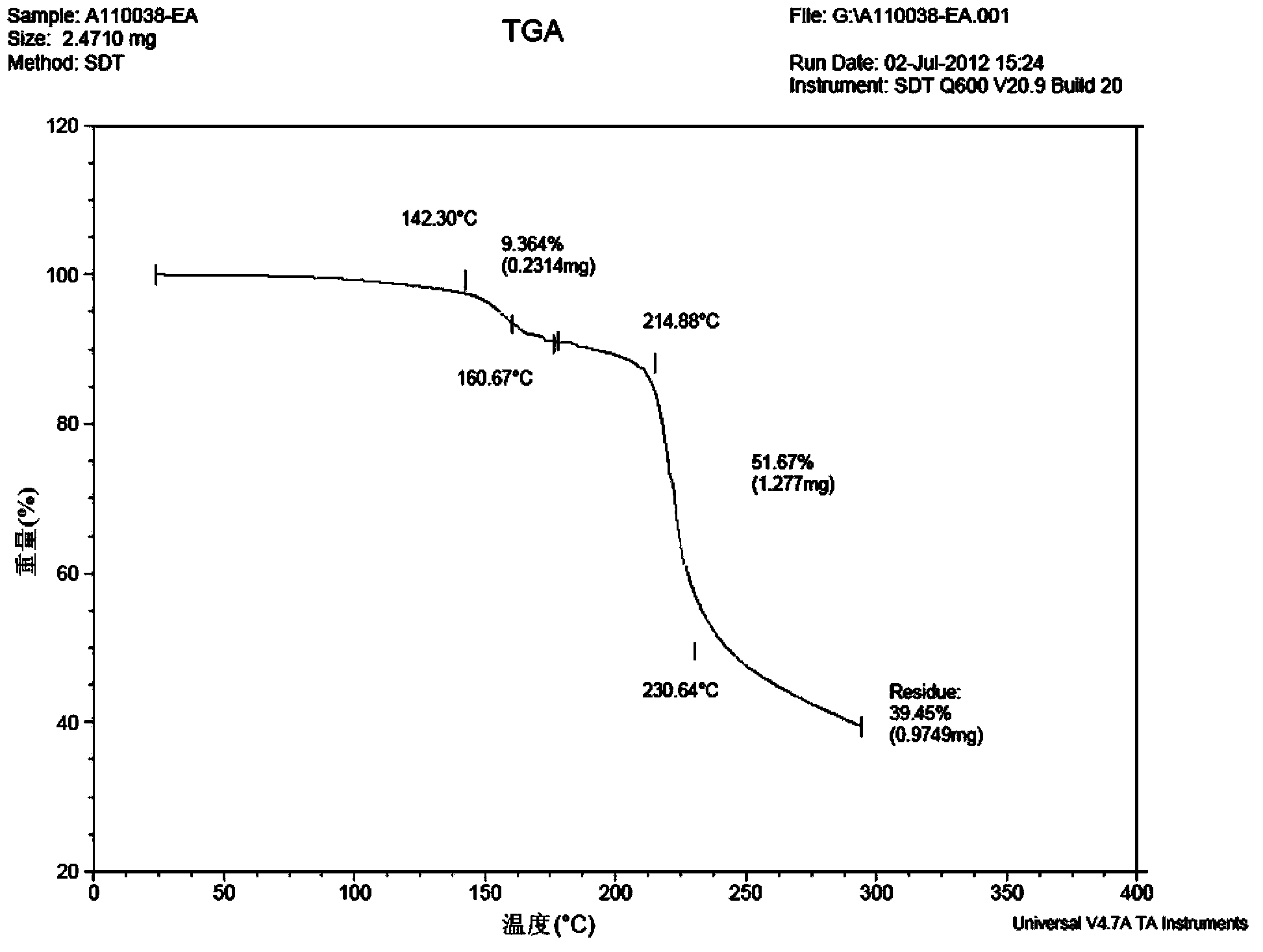
[0092] The HNMR data, PXRD spectrogram, DSC spectrogram and TGA spectrogram of the cabazitaxel solvate obtained in this embodiment are consistent with the spectrogram results obtained in Example 1 within the error range, and the stability test results are also consistent. It is consistent with the experimental results obtained in Example 1 within the error range.
Embodiment 3
[0094] Add 10.0g of cabazitaxel raw material (HPLC purity 85.3%) into 80mL ethyl acetate / tetrahydrofuran (5 / 1V / V), heat to 65°C, stir to dissolve completely; naturally cool to 50°C, add 0.1g of cabazitaxel Taxalate ethyl acetate solvate was used as crystal seed, kept stirring for 5 hours to precipitate crystals; then naturally cooled to 10°C, kept stirring for 2 hours; filtered, and the filter cake was washed with 20mL ethyl acetate / tetrahydrofuran (5 / 1V / V) Washing and vacuum drying at 40° C. for 8 hours to obtain 10.5 g of cabazitaxel ethyl acetate, which contained 9.07% ethyl acetate, and the HPLC purity was 99.6%.
[0095] The HNMR data, PXRD spectrogram, DSC spectrogram and TGA spectrogram of the cabazitaxel solvate obtained in this embodiment are consistent with the spectrogram results obtained in Example 1 within the error range, and the stability test results are also consistent. It is consistent with the experimental results obtained in Example 1 within the error rang...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com