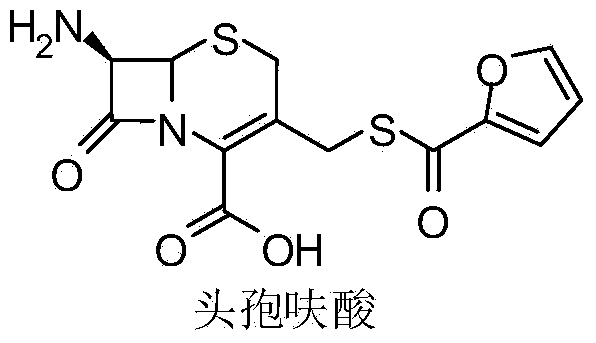
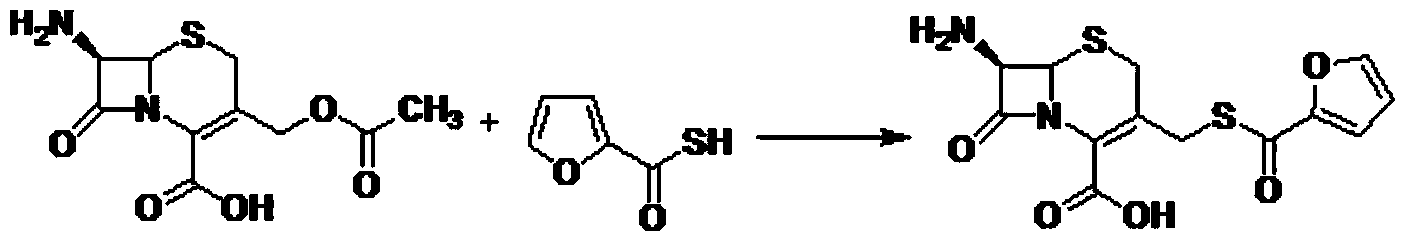
Preparation method of ceftiofur intermediate
A technology for ceftiofur and intermediates, which is applied in the field of preparation of ceftiofur intermediate cefuroxime, can solve the problems of difficult operation and high safety requirements for operators, and achieves reduced production cost, low cost and easy operability , the effect of optimizing the operating parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add water (2000mL) into the reaction flask, add sodium hydrosulfide (615.0g), stir until completely dissolved, cool to 5°C, add furoyl chloride (250.0g) dropwise, stir for 2 hours, add hydrochloric acid to adjust the pH value to 1.0, Ethyl acetate was added for extraction, and the organic phase was separated. Add anhydrous magnesium sulfate to dry. The yellow ethyl acetate solution was obtained by suction filtration, which was set aside.
Embodiment 2
[0025] The preparation of embodiment 2 furan-2-methylthiofuroic acid
[0026] Add water (1500mL) into the reaction flask, add sodium hydrosulfide (615.0g), stir until completely dissolved, cool to 0°C, add furoyl chloride (250.0g) dropwise, stir for 2 hours, add hydrochloric acid to adjust the pH value to 1.5, Add methyl tert-butyl ether for extraction, add an equal volume of saline to the organic phase, stir for half an hour, then separate the liquids to take the organic phase. Add anhydrous magnesium sulfate to dry. The yellow methyl tert-butyl ether solution was obtained by suction filtration, which was set aside.
Embodiment 3
[0027] The preparation of embodiment 3 furan-2-methylthiofuroic acid
[0028] Add water (1000mL) into the reaction flask, add sodium hydrosulfide (307.5g), stir until completely dissolved, cool to 10°C, add furoyl chloride (250.0g) dropwise, stir for 3h, add hydrochloric acid to adjust the pH value to 1.5, Dichloroethane was added for extraction, an equal volume of saline was added to the organic phase, and after stirring for half an hour, the organic phase was obtained by liquid separation. Add anhydrous sodium sulfate to dry. Suction filtration to obtain a yellow dichloroethane solution, set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com