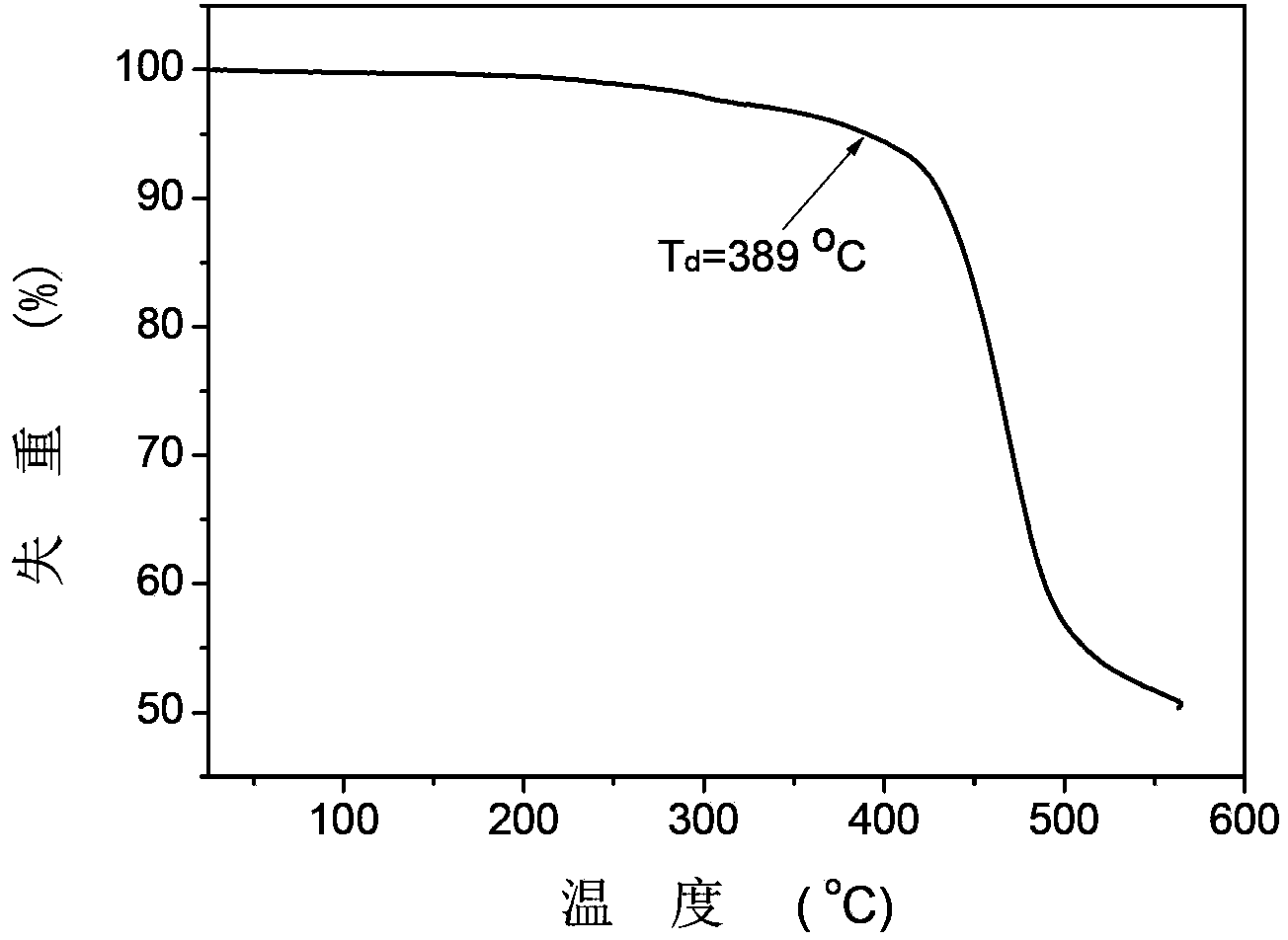
Bipolar red phosphorescent compound, and preparation method and application thereof
A compound and bipolar technology, applied in the field of bipolar red phosphorescent compounds and their preparation, can solve the problems of unfavorable carrier injection and transport balance, low glass transition temperature, lack of host materials, etc. Balanced carrier transport, good thermal stability, and reduced process flow effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the above-mentioned bipolar red light phosphorescent compound comprises the following steps:
[0028] S1, respectively provide compounds A and B represented by the following structural formula:
[0029] Namely (4-iodophenyl)triphenylsilane;
[0030] That is, 4-(diphenylamino)thiophenol;
[0031] S2. Under an oxygen-free environment (nitrogen, argon or a mixture of nitrogen and argon), dissolve compound A in an organic solvent, then add compound B, an inorganic base and a catalyst to obtain a mixed solution, and the mixed solution is at 70- Substitution reaction at 120°C for 3 to 12 hours, stop the reaction and cool to room temperature, the reaction solution is separated and purified to obtain the structural formula: Compound C (N,N-diphenyl-4-(4-(triphenylsilyl) phenylthio) aniline); the reaction formula is as follows:
[0032]
[0033] Preferably, the separation and purification treatment of compound C comprises steps:
[0034] The...
Embodiment 1
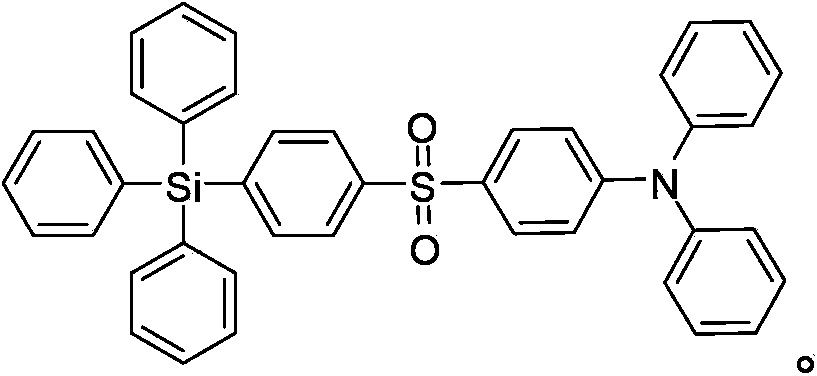
[0048]The preparation steps of the bipolar red phosphorescent compound of this example, namely N,N-diphenyl-4-(4-(triphenylsilyl)phenylsulfonylaniline) are as follows:
[0049]
[0050] Step 1: Dissolve (4-iodophenyl)triphenylsilane (36.7g, 80mmol) in 200mL N,N-dimethylformamide (DMF) solution under nitrogen protection, then add 4-(di Phenylamino)thiophenol (22.2g, 80mmol), potassium carbonate (22.1g, 160mmol), cuprous iodide (1.52g, 8mmol). The mixture was stirred at 120°C for 3 hours for substitution reaction. The reaction was stopped and cooled to room temperature, filtered, and the solid was washed three times with distilled water. The crude product was separated by silica gel chromatography using n-hexane as the eluent to obtain product C as an off-white solid. The yield was 87%.
[0051] Step 2: Dissolve C (24.5g, 40mmol) in 120mL of dichloromethane (DCM) solution in an ice-water mixing bath, and then drop it into 90mL of m-chlorobenzoic acid (mCPBA) in dichlorometh...
Embodiment 2
[0054] The preparation steps of the bipolar red phosphorescent compound of this example, namely N,N-diphenyl-4-(4-(triphenylsilyl)phenylsulfonylaniline) are as follows:
[0055]
[0056] Step 1: Dissolve (4-iodophenyl)triphenylsilane (36.7g, 80mmol) in 200mL toluene (Tol) solution under nitrogen protection, then add 4-(diphenylamino)thiophenol (24.4g, 88mmol), cesium carbonate (57.2g, 176mmol), copper powder (0.768g, 12mmol). The mixture was stirred for a substitution reaction at 110°C for 6 hours. The reaction was stopped and cooled to room temperature, filtered, and the solid was washed three times with distilled water. The crude product was separated by silica gel chromatography using n-hexane as the eluent to obtain product C as an off-white solid. The yield was 84%.
[0057] The second step: the difference between this step and the second step in Example 1 is that the oxidation reaction time is 18 hours; after the oxidation reaction finishes, add saturated NaHCO to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com