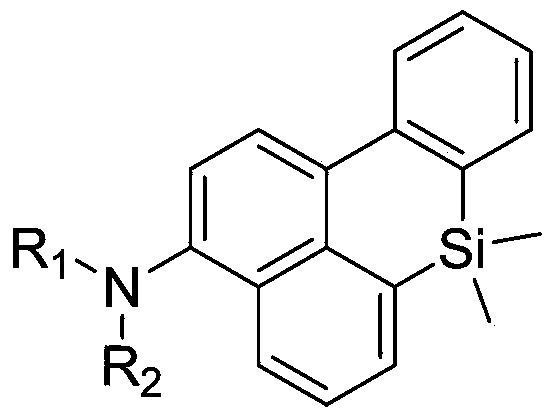
Silicious benzanthracene organic electroluminescent material, and preparation method and application thereof
A benzanthracene and luminescent technology, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that blue light materials cannot meet the development requirements of industrialization, and achieve high yield, improved solubility, and film formation good performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
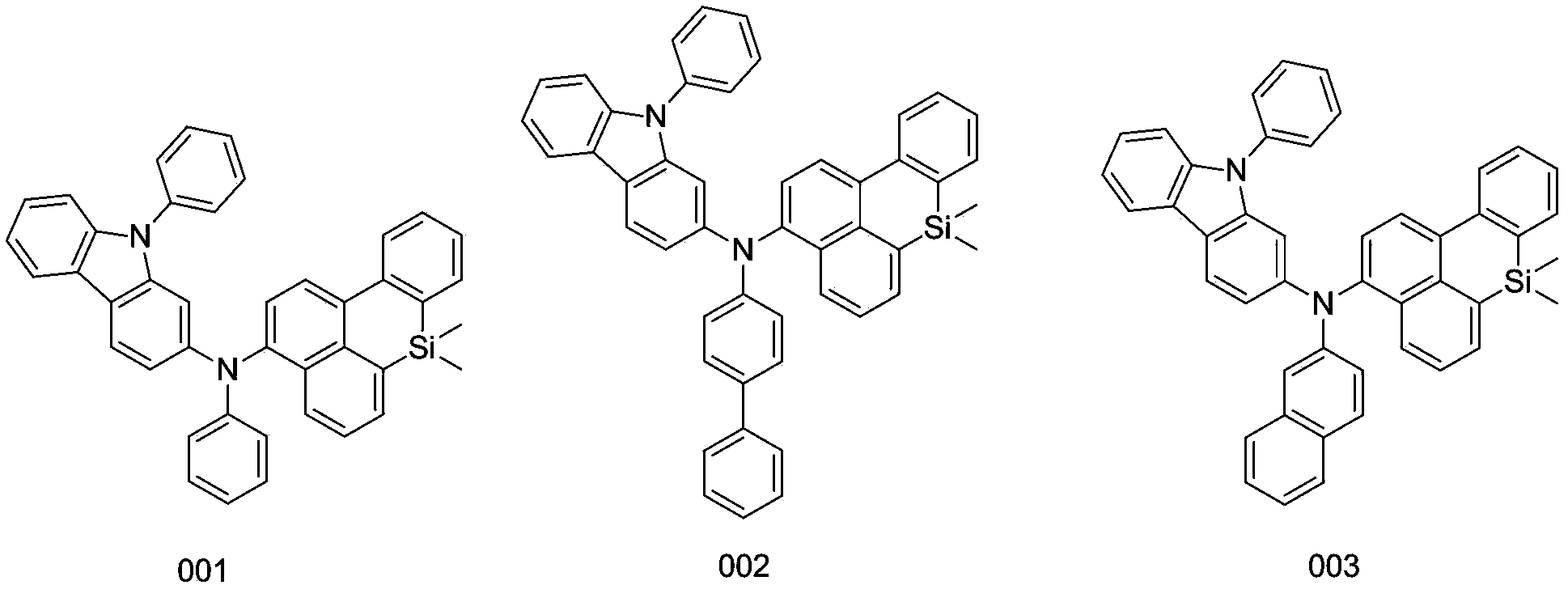
[0033] Embodiment 1: the synthesis of compound 001
[0034] The specific synthetic route is shown in the following formula:
[0035] Weigh 16.97g of silicon-containing benzanthracene bromine substitute, 42.05g of N-phenyl-2-carbazolylphenylamine, 12.32g of potassium tert-butoxide, 0.56g of palladium (II) acetate, tri-tert-butyl Phosphorus 0.51g was dissolved in 250ml of toluene, and reacted at 80°C for 6 hours under nitrogen protection. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, and the obtained solid was recrystallized with toluene. After drying, 27.86 g of yellow-white solid compound 001 was obtained, with a yield of more than 94% and an HPLC purity of more than 98%. Mass spectrum: calculated value 592.80; found value 592.78. Elemental analysis: Calculated value: C: 85.10%; H: 5.44%; N: 4.73%; Si: 4.74%; Tested value: C: 85.10%; H: 5.44%; N: 4.73%; Si: 4.74%.
Embodiment 2
[0036] Embodiment 2: the synthesis of compound 002
[0037] The specific synthetic route is shown in the following formula:
[0038] Weigh 16.97g of silicon-containing benzanthracene bromine substituent, 45.15g of N-phenyl-2-carbazolyl-p-biphenylamine, 12.32g of potassium tert-butoxide, 0.56g of palladium (II) acetate, tri-tert-butyl 0.51 g of base phosphorus was dissolved in 250 ml of toluene, and reacted at 86° C. for 8 hours under the protection of nitrogen. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, and the obtained solid was recrystallized with toluene. After drying, 31.43 g of yellow-white solid compound 002 was obtained, with a yield of more than 94% and an HPLC purity of more than 98%. Mass spectrum: calculated value 668.90; found value 668.88. Elemental analysis: calculated value C: 86.19%; H: 5.42%; N: 4.19%; Si: 4.20%; tested value C: 86.17%; H: 5.43%; N: 4.21%; Si: 4.19%.
Embodiment 3
[0039] Embodiment 3: the synthesis of compound 003
[0040] The specific synthetic route is shown in the following formula:
[0041]
[0042] Weigh 16.97g of silicon-containing benzanthracene bromine substitute, 46.38g of N-phenyl-2-carbazolylnaphthylamine, 12.32g of potassium tert-butoxide, 0.56g of palladium (II) acetate, tri-tert-butyl Phosphorus 0.51g was dissolved in 250ml of toluene, and reacted at 89°C for 8 hours under nitrogen protection. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, and the obtained solid was recrystallized from toluene. After drying, 29.89 g of yellow-white solid compound 003 was obtained, with a yield of more than 93% and an HPLC purity of more than 98%. Mass Spectrum: Calculated 642.86; Tested 642.84. Elemental analysis: Calculated value: C: 85.94%; H: 5.33%; N: 4.36%; Si: 4.37%; Test value: C: 85.92%; H: 5.34%; N: 4.36%; Si: 4.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com