Shenqi Fuzheng injection fingerprint atlas establishing method
A technology of Shenqi Fuzheng and fingerprints, which is applied in the field of establishment of fingerprints of Shenqi Fuzheng Injection, can solve the problems of long analysis time, complex processing, and inability to monitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] 1 Instruments and reagents
[0088] 1.1 Instrument: Agilent liquid mass spectrometer (1290UHPLC binary gradient pump, built-in vacuum degasser, 100-position autosampler, intelligent column oven, G6520B high-precision quadrupole tandem time-of-flight mass spectrometer system); Chromatographic column: Agilent Zorbax Eclipse Plus C18 (2.1×100 mm, 1.8 μm).
[0089] 1.2 Test drug: Shenqi Fuzheng Injection, provided by Limin Pharmaceutical Factory of Livzon Group. The reagents acetonitrile and formic acid used in the experiment were chromatographically pure, and the water was ultrapure water.
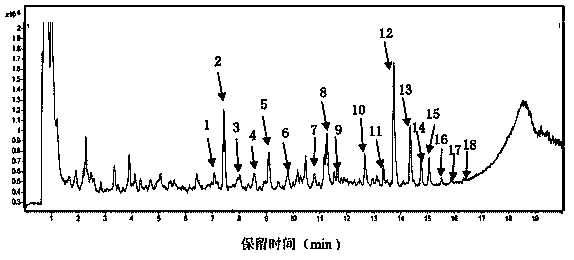
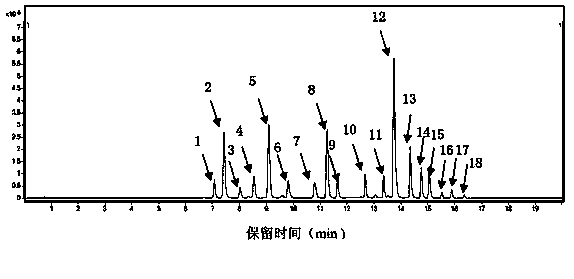
[0090] 2 Methods and Results
[0091] 2.1 Preparation of the test solution: Take Shenqi Fuzheng Injection and filter it with a 0.22um microporous membrane to obtain the solution.
[0092] 2.2 Preparation of mixed reference substance solution: Take appropriate amount of calycocoside glucoside reference substance and astragaloside IV reference substance, weigh them accurately, add met...
Embodiment 2
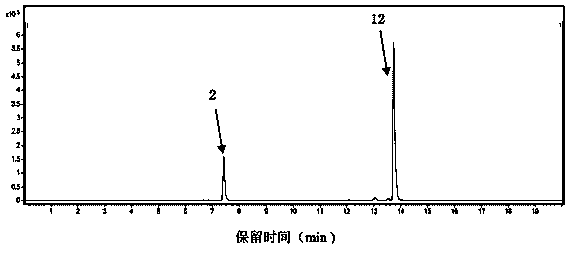
[0117] Example 2 Identification of Shenqi Fuzheng Injection
[0118] In recent years, with the increasing clinical use of Shenqi Fuzheng Injection, some criminals, driven by economic interests, use other varieties of counterfeit Shenqi Fuzheng Injection to sell and make huge profits, which has caused great harm to the brand of Shenqi Fuzheng Injection. Big negative impact brings very big economic loss to the enterprise of regular production and sales of Shenqi Fuzheng Injection. These counterfeit Shenqi Fuzheng injections are almost exactly the same as the genuine ones in appearance, and it is difficult to distinguish the authenticity from the fake.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com