Hemofiltration solution and preparation method thereof
A technology of hemofiltration and replacement fluid, applied in the fields of blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., can solve the problems of cumbersome operation without calcium replacement fluid, lack of restrictions on the widespread development of citrate anticoagulation, etc., to reduce death. rate, avoid hypophosphatemia and the loss of a large number of micronutrients, the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation of blood replacement fluid of the present invention
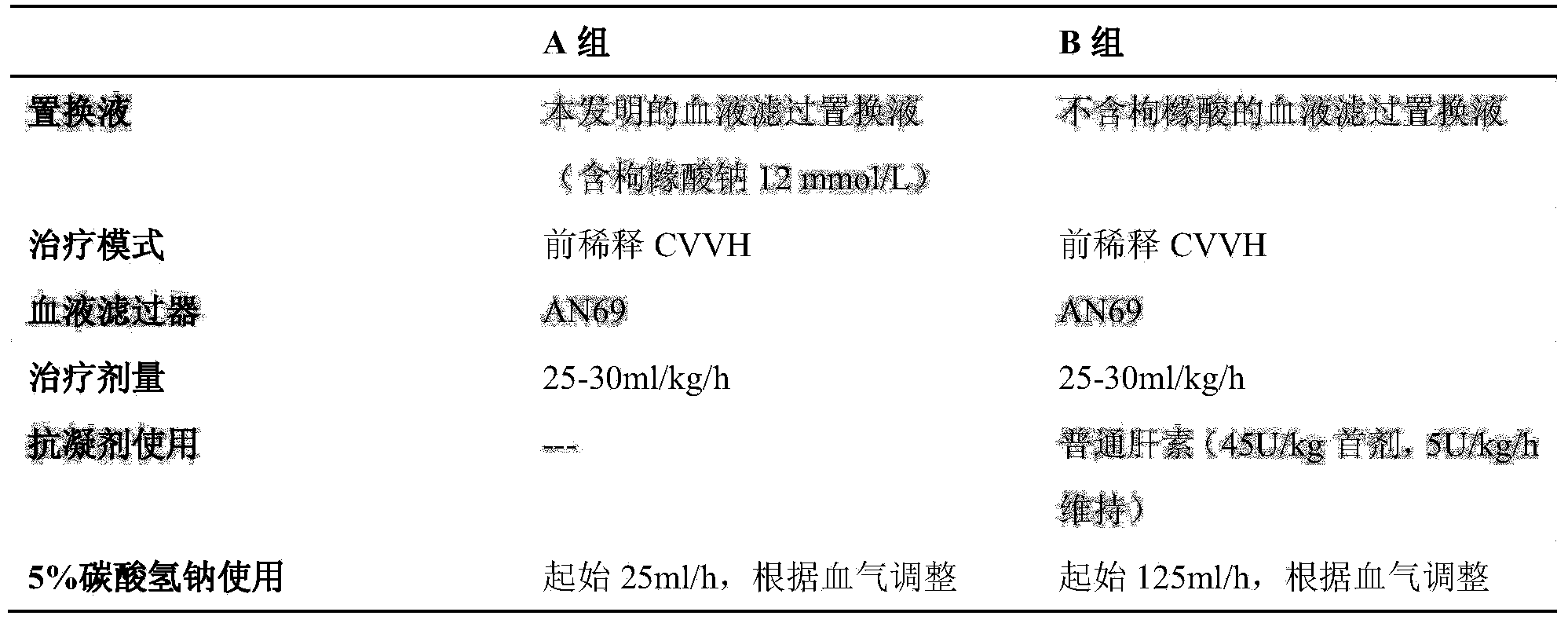
[0041] Take anhydrous glucose 2.880kg, sodium chloride 11.040kg, magnesium chloride hexahydrate 0.305kg, calcium chloride hexahydrate 0.613kg, sodium dihydrogen phosphate dihydrate 0.312kg, sodium citrate dihydrate 7.056kg, vitamin C 35.200g, Folic acid 0.035g, vitamin B6 0.034g, sodium selenite 0.346g, dissolve in appropriate amount of water for injection, add appropriate amount of activated carbon and stir, filter through a 0.45μm titanium rod, add water for injection to a total volume of 2000 liters, filter through a 0.22μm terminal filter , filled in the infusion bag of non-PVC composite film soft bag, sealed, and sterilized at 115 degrees Celsius for 30 minutes.
[0042] Corresponding ion concentration:
[0043] Sodium ion (mmol / L) 130 Magnesium ion (mmol / L) 0.75 Potassium ion (mmol / L) 0 Calcium ion (mmol / L) 1.4 Chloride ion (mmol / L) 100 Dihydrogen...
Embodiment 2
[0044] Embodiment 2 Preparation of blood replacement fluid of the present invention
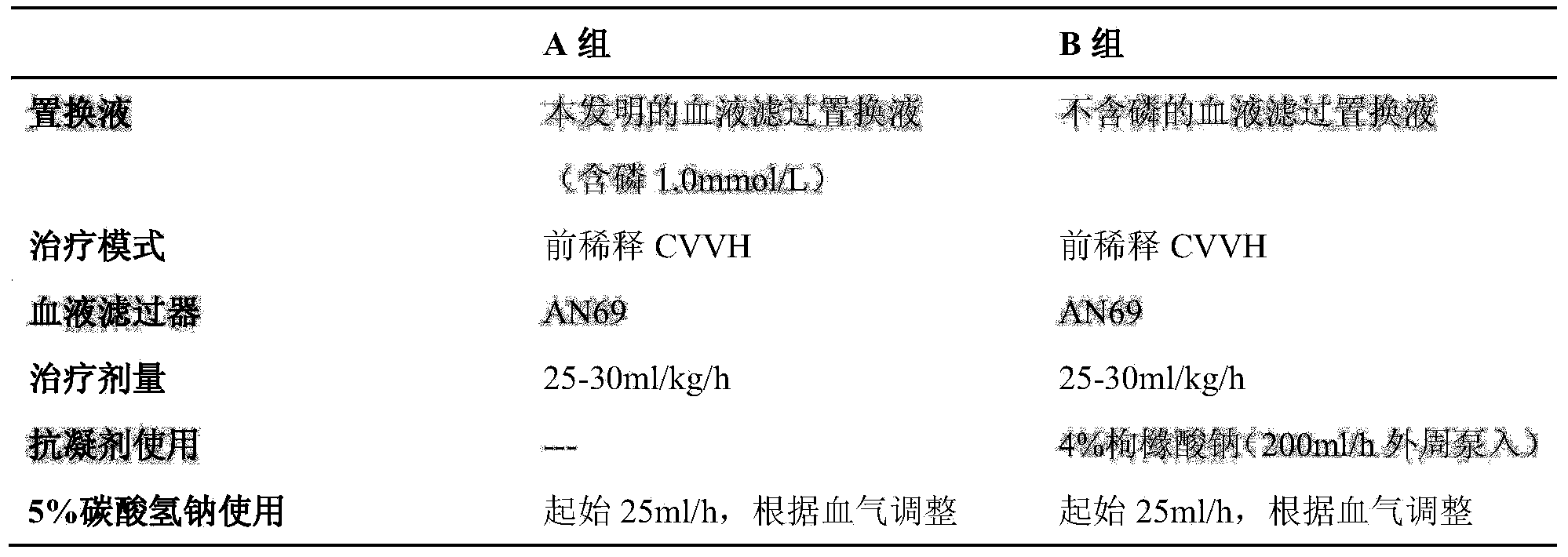
[0045] Take anhydrous glucose 1.800kg, sodium chloride 10.432kg, magnesium chloride hexahydrate 0.203kg, calcium chloride hexahydrate 0.438kg, potassium chloride 0.298kg, sodium dihydrogen phosphate dihydrate 0.208kg, sodium citrate dihydrate 9.408kg , sodium selenite 0.346g, dissolved in an appropriate amount of water for injection, added an appropriate amount of activated carbon and stirred, filtered through a 0.45μm titanium rod, added water for injection to a total volume of 2000 liters, filtered through a 0.22μm terminal filter, and filled in a non-PVC composite membrane Put it in the infusion bag of the soft bag, seal it, and sterilize it at 115 degrees Celsius for 30 minutes.
[0046] Corresponding ion concentration:
[0047] Sodium ion (mmol / L) 125 Magnesium ion (mmol / L) 0.75 Calcium ion (mmol / L) 1.0 Potassium ion (mmol / L) 2 Chloride ion (mmol / L) 92....
Embodiment 3
[0048] Embodiment 3 Preparation of hemofiltration replacement fluid of the present invention
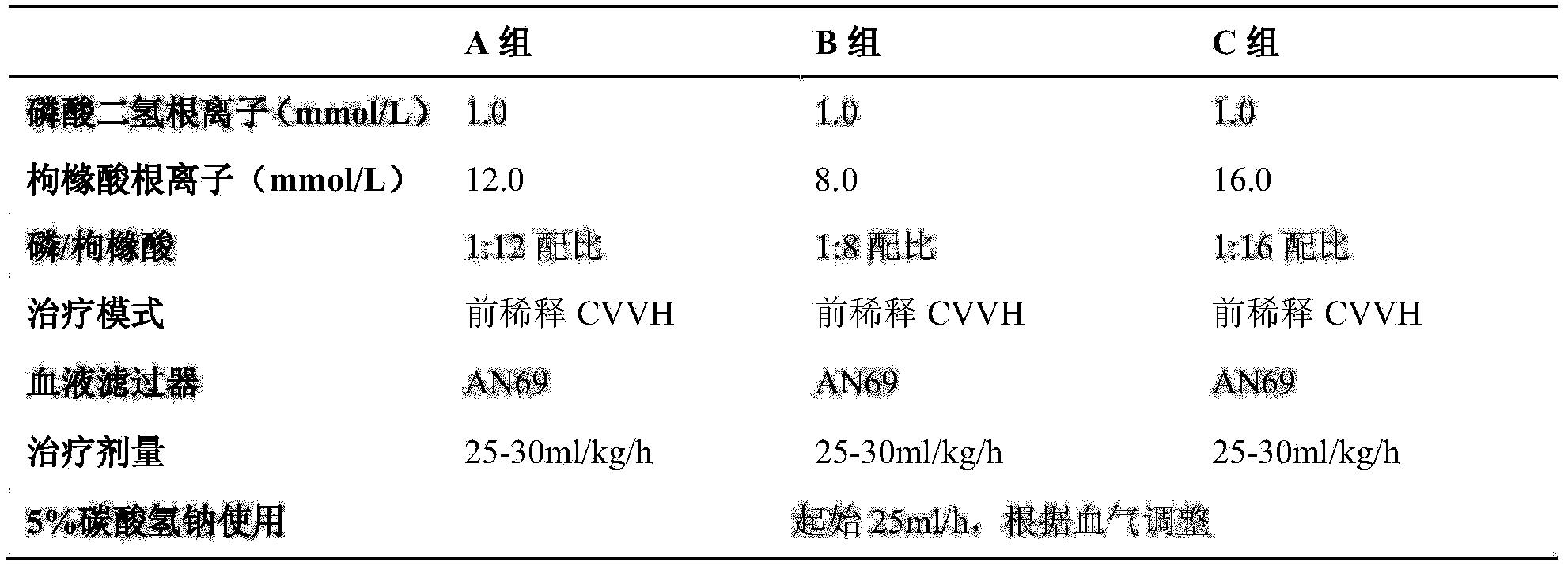
[0049] Anhydrous glucose 4.320kg, sodium chloride 12.740kg, magnesium chloride hexahydrate 0.203kg, calcium chloride hexahydrate 0.745kg, potassium chloride 0.373kg, sodium dihydrogen phosphate dihydrate 0.468kg, sodium citrate dihydrate 4.704kg, Dissolve 52.800g of vitamin C, 0.070g of folic acid, and 0.027g of vitamin B in an appropriate amount of water for injection, add an appropriate amount of activated carbon and stir, filter through a 0.45μm titanium rod, add water for injection to a total volume of 2000 liters, filter through a 0.22μm terminal filter, and fill Put it in the infusion bag of non-PVC composite film soft bag, seal it, and sterilize it at 115 degrees Celsius for 30 minutes.
[0050] Corresponding ion concentration:
[0051] Sodium ion (mmol / L) 145 Magnesium ion (mmol / L) 0.5 Potassium ion (mmol / L) 2.5 Calcium ion (mmol / L) 1.7 Chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com