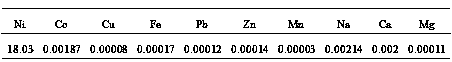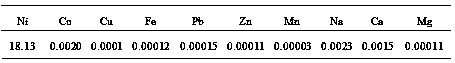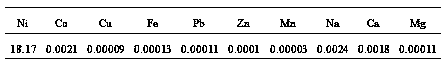Method for preparing nickel aminosulfonate with solid sulfamic acid
A technology of nickel sulfamic acid and sulfamic acid, applied in the direction of sulfamic acid, nitrogen and non-metallic compounds, etc., can solve the problems of low nickel content in nickel sulfamate solution, increase production process, increase water consumption, etc. Achieve the effects of shortening the production cycle, reducing the acid-dissolving process, and reducing steam consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Take cobalt sulfate raffinate (containing crude nickel sulfate) as raw material, remove impurities to obtain nickel-containing raw material solution, nickel content: 47.5g / L;
[0027] 2) React the organic phase of P204 and diluent (ratio: 1:3) with 35% sodium hydroxide solution to make sodium soap, with a saponification rate of 75%;
[0028] 3) reacting the nickel-containing raw material solution in step 1 with the sodium soap prepared in step 2 to obtain a nickel-loaded organic phase;
[0029] 4) Wash the nickel-loaded organic phase with 0.7g / L sulfuric acid, and the O / A ratio is 10:1;
[0030] 5) Wash the nickel-loaded organic phase with 5g / L sulfamic acid, the ratio of O / A is 1:1, and put the loaded organic phase into the high-level storage tank after washing;
[0031] 6) Put the solid sulfamic acid into the extraction reactor, put 2L of pure water into the organic clarification tank, and the nickel-loaded organic phase obtained in step 5 is also put into the org...
Embodiment 2
[0036] 1) The thick nickel hydroxide solid is leached with sulfuric acid to obtain a nickel sulfate leaching solution, which is filtered and removed to obtain a nickel-containing raw material solution containing 52.3g / L of nickel.
[0037] 2) React the organic phase of P204 and diluent (ratio 1:3) with 32% sodium hydroxide solution to make sodium soap, with a saponification rate of 75%;
[0038] 3) reacting the raw material solution in step 1 with the sodium soap prepared in step 2 to obtain a nickel-loaded organic phase;
[0039] 4) Wash the nickel-loaded organic phase with 0.85g / L sulfuric acid, and the O / A ratio is 11:1;
[0040] 5) Wash the nickel-loaded organic phase with 10g / L sulfamic acid, the ratio of O / A is 3:1, and put the loaded organic phase into the high-level storage tank after washing;
[0041] 6) Put the solid sulfamic acid into the extraction reactor, put 3L pure water into the organic clarifier, and the nickel-loaded organic phase obtained in step 5 is also...
Embodiment 3
[0046] 1) Take copper sulfate raffinate (containing crude nickel sulfate) as raw material, remove impurities, and obtain nickel-containing raw material solution containing nickel: 51.5g / L;
[0047] 2) The organic phase of P204 and diluent (1:3.5 ratio) is reacted with 45% sodium hydroxide solution to make sodium soap, and the saponification rate is 90%;
[0048] 3) reacting the raw material solution in step 1 with the sodium soap prepared in step 2 to obtain a nickel-loaded organic phase;
[0049] 4) Wash the nickel-loaded organic phase with 0.65g / L sulfuric acid, and the O / A ratio is 12:1;
[0050] 5) Wash the nickel-loaded organic phase with 6g / L sulfamic acid, the ratio of O / A is 3:1, and put the loaded organic phase into the high-level storage tank after washing;
[0051] 6) Put the solid sulfamic acid into the extraction reactor, put 3L pure water into the organic clarifier, and the nickel-loaded organic phase obtained in step 5 is also put into the organic clarifier thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com