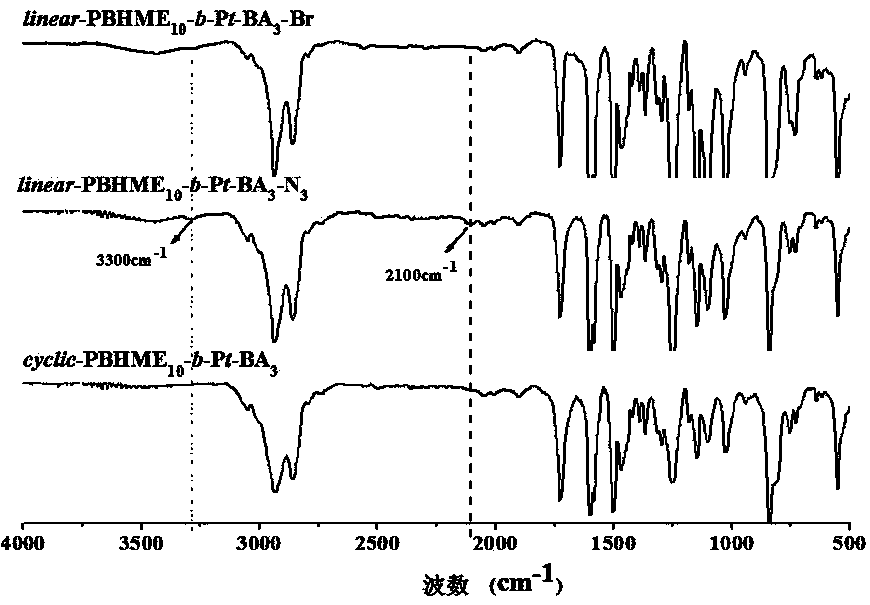
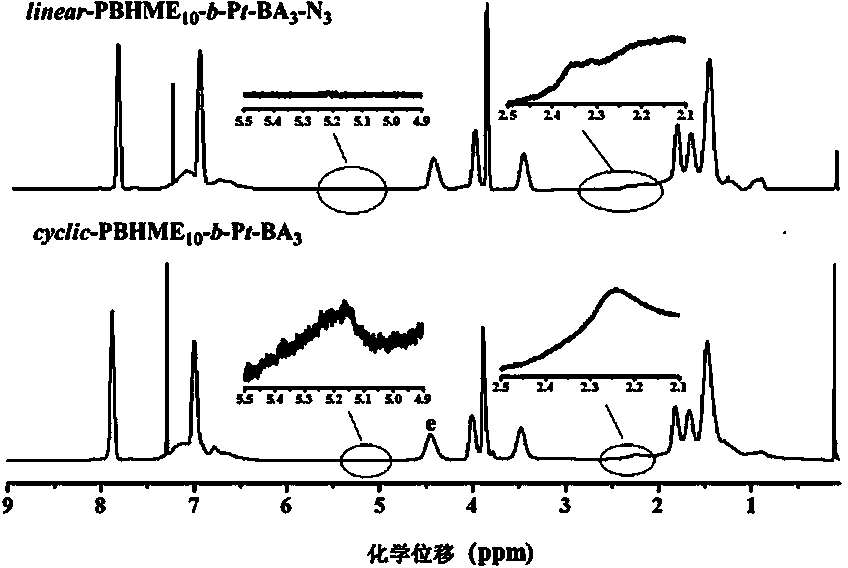
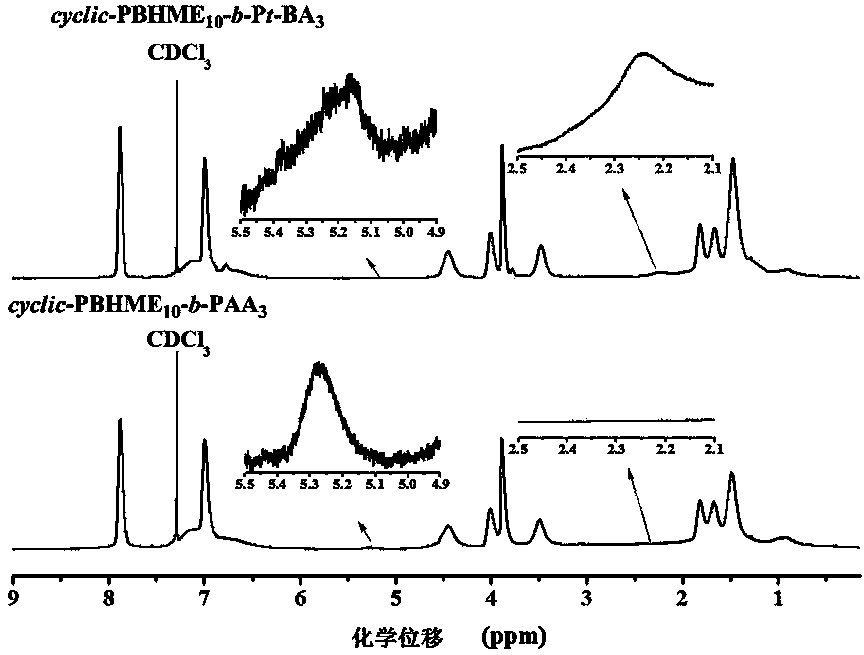
Cyclic azobenzene amphiphilic segmented copolymer and its preparation method
An amphiphilic block and cyclic azobenzene technology, which is applied in organic chemistry and other fields, can solve the problems of difficult synthesis and purification structure characterization, and achieve the effect of narrow molecular weight distribution, small difference, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment one: Cyclic Azobenzene Amphiphilic Block Copolymer cycle -PBHME 10 - b -PAA 3 Synthesis
[0062] (1) Synthesis of side-chain azobenzene functional monomer: In a 100mL beaker, add 30mL of concentrated hydrochloric acid and 20mL of deionized water, add p-methoxyaniline (6.16g) under stirring, and cool the mixture to 3 °C; NaNO 2 (3.50g) was dissolved in 15mL deionized water and added dropwise to the above mixture. After the dropwise addition was completed, continue stirring at 3°C for 1 hour to obtain a diazotized solution of methoxyaniline;
[0063] Add phenol (4.70g), water 100mL and NaOH (7.00g) into a 250mL beaker, stir evenly, cool in an ice-water bath until the solution temperature is 0°C, add the obtained diazotization solution dropwise to the phenol solution, and control the reaction solution The temperature is at 0°C. After the dropwise addition, continue to stir at 0°C for 1 h, then stir at room temperature for 2 h, filter with suction, was...
Embodiment 2
[0104] Embodiment two: Cyclic Azobenzene Amphiphilic Block Copolymer cycle -PBHME 7 - b -PAA 4 Synthesis
[0105] (1) Synthesis of side-chain azobenzene functional monomers: In a 100mL beaker, add 30mL of concentrated hydrochloric acid and 20mL of deionized water, add p-methoxyaniline (6.16g) under stirring, and cool the mixture to 5 °C; NaNO 2 (3.50g) was dissolved in 15mL deionized water and added dropwise to the above mixture. After the dropwise addition was completed, continue stirring at 5°C for 1 hour to obtain a diazotized solution of methoxyaniline;
[0106] Add phenol (4.70g), water 100mL and NaOH (7.00g) into a 250mL beaker, stir evenly, cool in an ice-water bath until the solution temperature is 5°C, add the obtained diazotization solution dropwise to the phenol solution, and control the reaction solution The temperature is at 5°C. After the dropwise addition, continue to stir at 5°C for 1 h, then stir at room temperature for 2 h, filter with suction, wash ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com