Reagent and method for visual lamp detection of prawn taura syndrome virus
A technology of syndrome virus and detection reagent, applied in the field of warm amplification detection, to achieve the effect of improved sensitivity, simple instrument and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
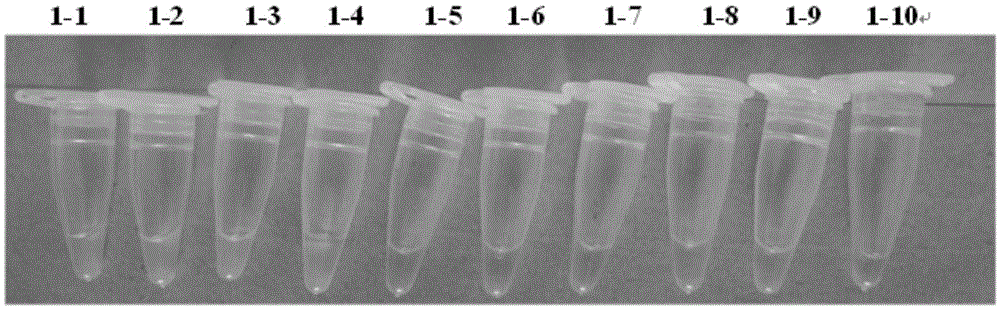
[0041] Embodiment 1: specificity test
[0042] Step (1) Sample RNA extraction: Take about 100 mg of the hepatopancreas, gills, and muscle tissue of the prawns for tissue homogenization, and then take an appropriate amount of samples, use Trizol RNA extraction reagent (GIBCOBRL, USA) to extract the total RNA of the prawns, and place in Store at -20°C for later use.
[0043] Step (2) Preparation of LAMP detection liquid system
[0044] ①: For the capsid protein gene sequence in the conserved region of the shrimp taura syndrome virus genome, design 6 specific amplification primers, including two outer primers F3 and B3, two inner primers FIP and BIP, and two loop primers LF and LB.
[0045] F3 is 5'-GGTCCTGGACTTCAAACA-3';
[0046] B3 is 5'-TTGCGTGGTGGGACTA-3';
[0047] FIP is 5'-AGGTTCCACAATCTTGATGAATGAT-CTGTTACATTCTTAGACAGCACTA-3';
[0048] BIP is 5'-TCACTGTTAGTAACACTACCTCCT-CCTGCTAACCCAGTCGA-3';
[0049] LF is 5'-ATCCCAATCACTAATCAGAATG-3';
[0050] LB is 5'-AGTCCTCCACT...
Embodiment 2
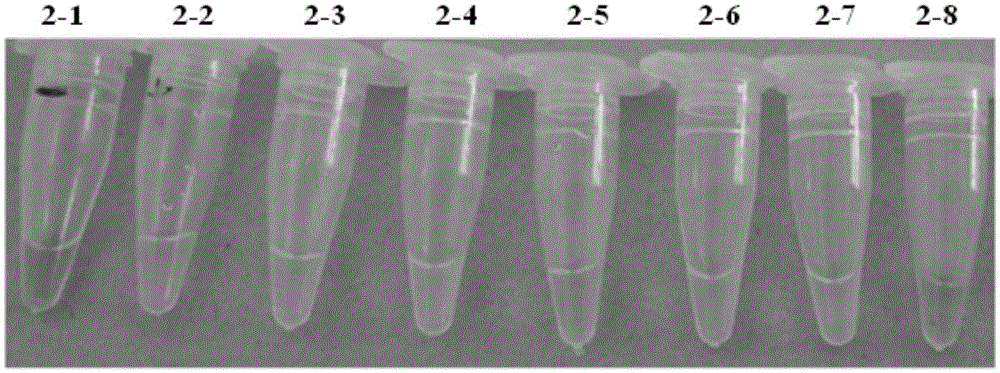
[0061] Embodiment 2: specificity test
[0062] The difference between this example and Example 1 lies in the various reagents required in the preparation of the LAMP detection liquid system in step (2) LAMP detection liquid system for shrimp taura syndrome virus, and the addition amounts and concentrations of the reagents are as follows:
[0063]0.5 μL 10 μmol / L F3 (final concentration 0.2 μmol / L), 0.5 μL 10 μmol / L B3 (final concentration 0.2 μmol / L), 2.0 μL 100 μmol / L FIP (final concentration 8 μmol / L), 2.0 μL 100 μmol / L BIP (final concentration 8 μmol / L), 0.5 μL 10 μmol / LLF (final concentration 0.2 μmol / L), 0.5 μL 10 μmol / L LB (final concentration 0.2 μmol / L), 2.5 μL 10× reaction buffer BstDNA polymerase buffer (final concentration 1×), 2.5 μL 40 U / μL strand-displacing DNA polymerase (final concentration 4 U), 2.5 μL 100 mmol / L MgSO 4 (final concentration 10mmol / L), 0.5μL 1mmol / L MnCl 2 (final concentration 0.02mmol / L), 1μL 25mmol / L betaine (final concentration 1.0mmol / L...
Embodiment 3
[0066] Embodiment 3: specificity test
[0067] The difference between this example and Example 1 lies in the various reagents required in the preparation of the LAMP detection liquid system in step (2) LAMP detection liquid system for shrimp taura syndrome virus, and the addition amounts and concentrations of the reagents are as follows:
[0068] 1.0 μL 200 μmol / L F3 (final concentration 8 μmol / L), 1.0 μL 200 μmol / L B3 (final concentration 8 μmol / L), 2.0 μL 2.5 μmol / L FIP (final concentration 0.2 μmol / L), 2.0 μL 2.5 μmol / L L of BIP (final concentration 0.2 μmol / L), 1.0 μL 200 μmol / LLF (final concentration 8 μmol / L), 1.0 μL 200 μmol / L LB (final concentration 8 μmol / L), 2.5 μL 10× reaction buffer BstDNA polymerase buffer (final concentration 1× ), 2.5 μL 40U / μL strand displacement DNA polymerase (final concentration 4U), 1.0 μL 20mmol / L MgSO 4 (final concentration 0.8mmol / L), 1.25μL of 25mmol / L MnCl 2 (final concentration 1.25mmol / L), 1μL 1mmol / L betaine (final concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com