Sterilizing composition containing benzothiostrobin and epoxiconazole
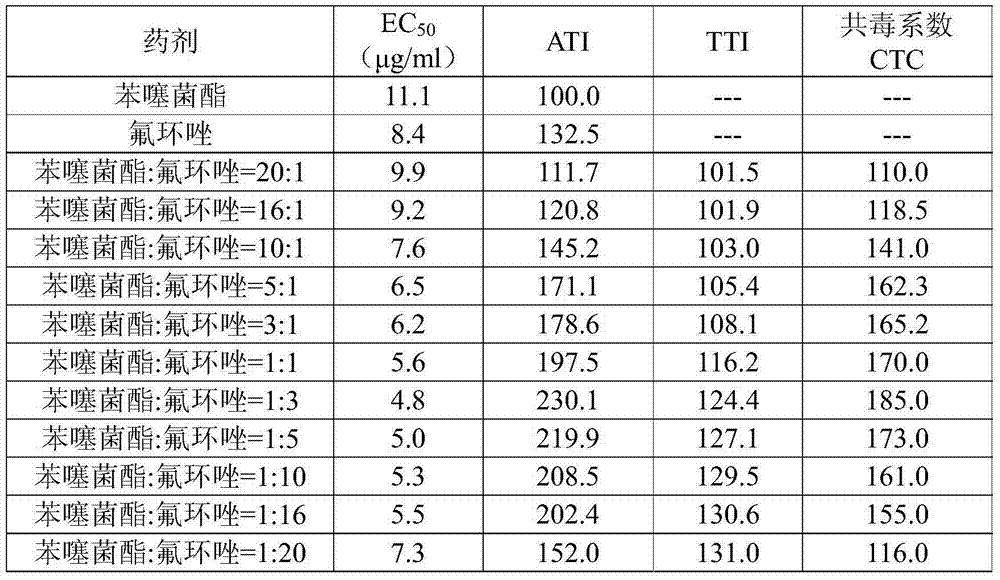
一种苯噻菌酯、氟环唑的技术,应用在农药领域,能够解决未发现苯噻菌酯与氟环唑复配、不良化学反应、组合物毒性等问题,达到显著协同增效作用、提高药效、存储和运输方便的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
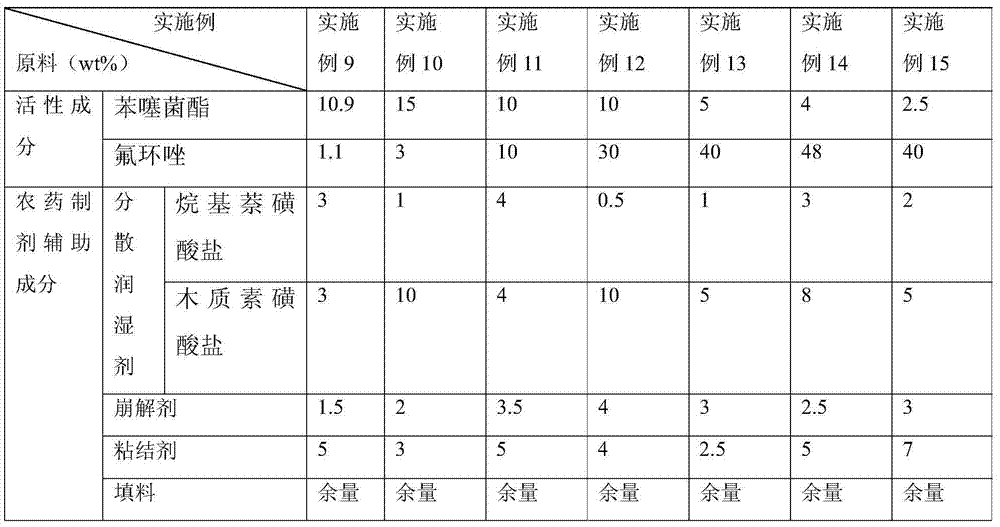
Examples
Embodiment 122
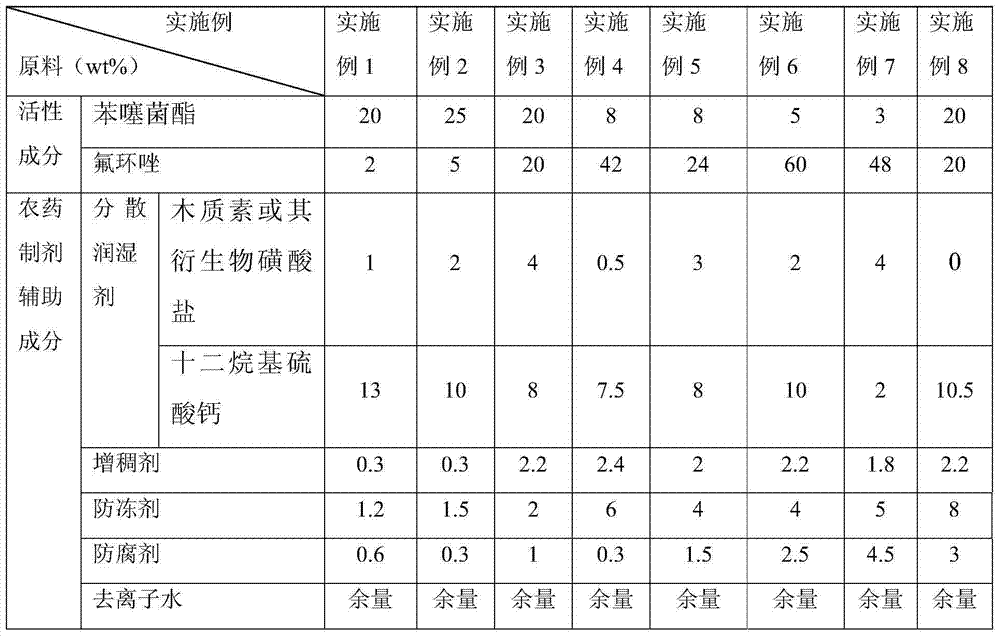
[0030] Embodiment 122% pentothiastrobin · epoxiconazole suspension concentrate
[0031] The raw material formula is shown in Table 1, wherein the thickener is gum arabic, the antifreeze is propylene glycol, and the preservative is formaldehyde.
[0032] The preparation method of the suspending agent is as follows: add each raw material into the sand mill cylinder according to the formula, and after stirring evenly, pre-disperse it with a high shear machine, then transfer it to a sand mill for sand milling, add sanding medium zirconia beads, and start sanding Grinding, so that the average particle size of the material is below 3 μm, stop sanding after 5 hours, filter, remove the zirconia beads, and obtain.
[0033] The technical indicators of the obtained suspension concentrate are as follows: Suspension rate ≥ 90.0%; pH value 6.0-8.0; sieve analysis (passing through a 75 μm sieve) ≥ 98%; persistent foaming (after 1 min) ≤ 25ml; pouring property: residue after pouring ≤4.0%, r...
Embodiment 230
[0034] Embodiment 230% pentothiastrobin · epoxiconazole suspension concentrate
[0035] The raw material formula is shown in Table 1, wherein the thickener is xanthan gum, the antifreeze is ethylene glycol, and the preservative is sodium sorbitol.
[0036] The preparation method of the suspension is the same as in Example 1, and the technical indicators of the obtained suspension are as follows: suspension rate ≥ 91.5%; pH value 6.0-8.0; sieve analysis (through a 75 μm sieve) ≥ 98%; persistent foaming (after 1min) ≤ 25ml; Pourability: residue after pouring ≤3.0%, residue after washing ≤0.5%; suspension rate ≥87% after hot storage at 54±2°C for 30 days; suspension rate ≥87% after cold storage at 0±2°C for 30 days.
Embodiment 340
[0037] Embodiment 340% pentothiastrobin · epoxiconazole suspension concentrate
[0038] The raw material formula is shown in Table 1, wherein the thickener is xanthan gum and methylcellulose (weight ratio 0.2:2), the antifreeze is isopropanol, and the preservative is sodium sorbitol.
[0039] The preparation method of the suspension is the same as in Example 1, and the technical indicators of the obtained suspension are as follows: suspension rate ≥ 93.5%; pH value 6.0-8.0; sieve analysis (through a 75 μm sieve) ≥ 98%; persistent foaming (after 1min) ≤ 25ml; Pourability: residue after pouring ≤3.0%, residue after washing ≤0.5%; suspension rate ≥90% after hot storage at 54±2°C for 30 days; suspension rate ≥90% after cold storage at 0±2°C for 30 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com