A kind of synthetic technique of vinpocetine related impurity a
A technology of vinpocetine and synthesis process is applied in the field of synthesis of polycyclic indole alkaloid raw material vinpocetine related impurity A, which can solve the problems of difficult separation of products, small production amount, etc. Simple equipment and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
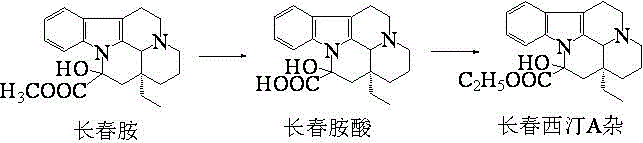
[0015] The synthesis of embodiment 1 vincine
[0016] In a 500 mL three-necked flask, sequentially add 300 mL of solvent 70% ethanol-water solution, 20 g of reaction raw material vincamine, and 10 g of basic catalyst anhydrous potassium carbonate to make the pH of the system around 12, slowly raise the temperature to 50 °C, and react 9 h, Thin-layer chromatography monitors the complete reaction of the raw material vincamine, which is considered as the completion of the hydrolysis reaction. Then, stir and cool down to below 10°C, adjust the pH value to 6 with 1% dilute hydrochloric acid, and precipitate a white amorphous solid, which is the product vinblastine. After drying, 19.1 g of vinblastine product was obtained for use, with a weight yield of 95.5%.
Embodiment 2
[0017] Example 2 The preparation of Vinpocetine A complex 1
[0018] In a 500 mL three-neck flask, stir to lower the temperature below 5 °C, add 300 mL of absolute ethanol and 1.8 g of anhydrous p-toluenesulfonic acid in sequence, stir and dissolve, then add 18.0 g of dry vinblastine, and stir the system at 30 The reaction was carried out at ℃ for 6 h, and the complete reaction of the raw material vinblastine was monitored by thin-layer chromatography, and the ethyl esterification reaction was considered complete. Then the temperature was lowered to below 10°C, and the pH of the reaction solution was adjusted to about 7 with 5% alkali solution, which was regarded as the completion of the vinpocetine A hybrid synthesis step. Transfer the vinpocetine A mixed ethanol solution of the previous step into the concentration bottle, and concentrate under reduced pressure to recover ethanol. Then add dichloromethane and water, transfer to a separatory funnel for extraction twice, add 1...
Embodiment 3
[0019] Embodiment 3 Preparation of Vinpocetine A complex 2
[0020] In a 500 mL three-necked flask, stir to lower the temperature below 15 °C, add 300 mL of absolute ethanol, 5.0 g of N,N-dicyclohexyl imine and 3.5 g of 4-dimethylaminopyridine in sequence, stir to dissolve, then add dry 18.0 g of vinblastine, the system was stirred at 40 °C for 4 h, and the complete reaction of the raw material vincine was monitored by thin-layer chromatography, and the ethyl esterification reaction was considered complete. That is, the hybrid synthesis step of Vinpocetine A is completed. Transfer the vinpocetine A mixed ethanol solution of the previous step into the concentration bottle, and concentrate under reduced pressure to recover ethanol. Then add dichloromethane and water, transfer to a separatory funnel for extraction twice, add 150 mL of dichloromethane each time, combine the organic phases after extraction, and dry over anhydrous sodium sulfate. Then transfer to the concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com