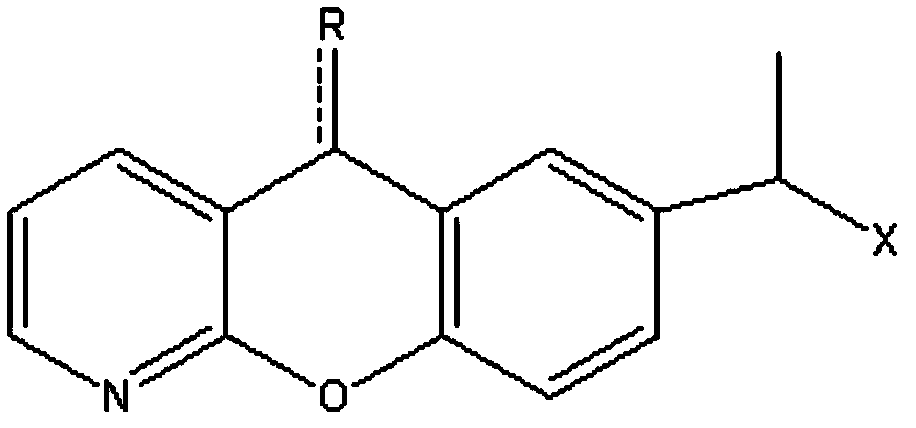
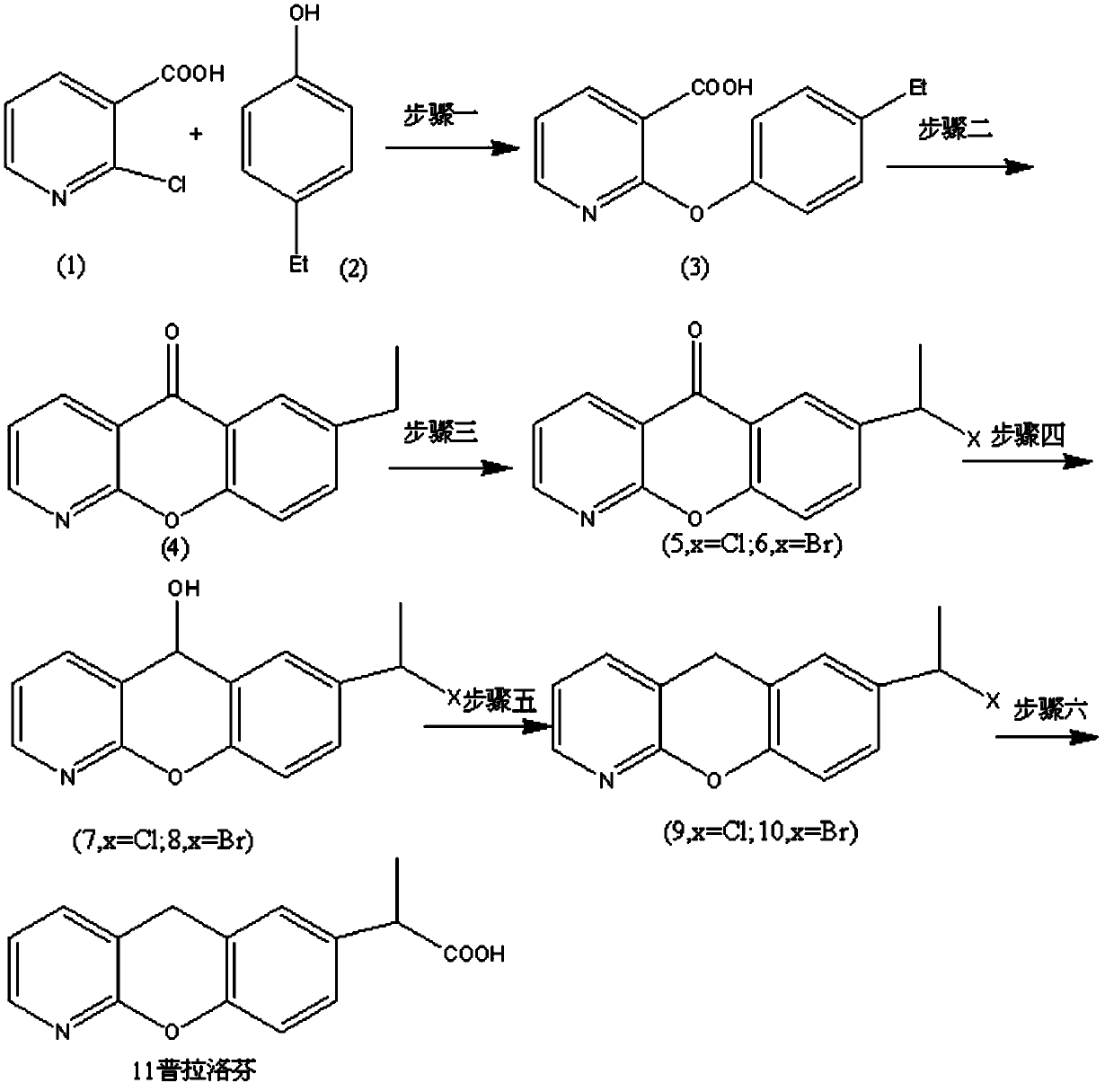
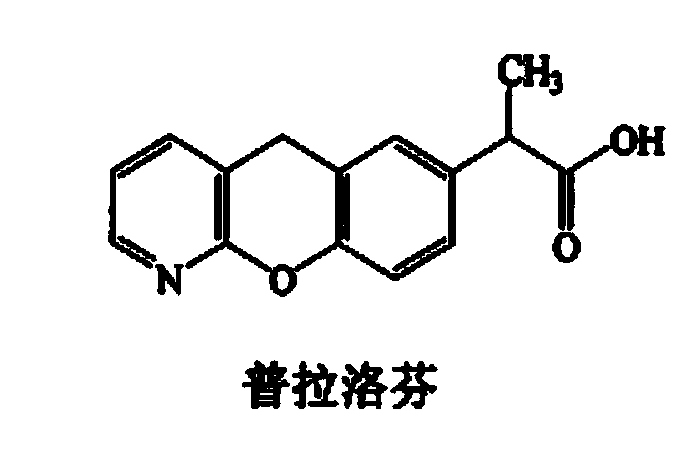
Synthetic method for pranoprofen
A technology for compounds and intermediates, applied in the field of propionic acid non-steroidal anti-inflammatory drugs, can solve the problems of reduced yield, unreacted solidification, etc., and achieves the effects of shortening synthesis steps, improving raw material utilization, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 12-(4-ethylphenoxy)nicotinic acid (3)
Embodiment 1a
[0045] Example 1a: Add 51g of sodium hydroxide and 600mL of methanol into a 1000mL three-neck flask, stir mechanically until completely dissolved, then add 232g of 4-ethylphenol in batches, and add 100g of 2-chloronicotinic acid after cooling down to room temperature. The reaction mixture is heated to evaporate most of the methanol, and then continue to heat to 170~180 o C, after removing all low boilers, heat preservation reaction for 1.5 hours. After cooling down to room temperature, 1000 mL of water was added to dissolve the residue, and extracted with ethyl acetate (300 mL×3). The aqueous phase was collected and the pH was adjusted to about 4 with 6N HCl aqueous solution, and a large amount of solids precipitated out of the system. Suction filtration, washing with water, and drying of the filter cake gave 138 g of light brown solid 3, with a yield of 90%.
Embodiment 1b
[0046] Example 1b: Add 51g of potassium hydroxide and 600mL of absolute ethanol into a 1000mL three-necked flask, stir mechanically until completely dissolved, add 232g of 4-ethylphenol in batches, and add 100g of 2-chloronicotinic acid after cooling down to room temperature. The reaction mixture is heated to steam most of the ethanol, and then heated to 200 oC, after removing all low boilers, heat preservation reaction for 2 hours. After cooling down to room temperature, 1000 mL of water was added to dissolve the residue, and extracted with ethyl acetate (300 mL×3). The aqueous phase was collected and the pH was adjusted to about 5 with aqueous acetic acid, and a large amount of solids precipitated out of the system. Suction filtration, washing with water, and drying of the filter cake gave 130 g of brown solid 3, with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com