Detection kit for human herpes viruses EBV and VZV
A detection kit and technology for human herpes are applied in the field of human herpes virus EBV and VZV detection kits, which can solve the problems of low antibody specificity, long time, and low sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
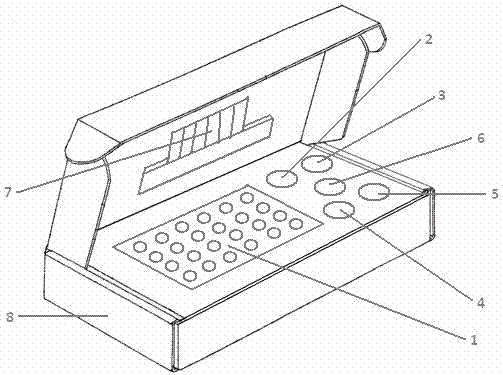
[0028] see figure 1 , the human herpesvirus EBV and VZV detection kit provided by the present invention is composed of quantitative PCR reaction solution 1, EBV standard substance 2, VZV standard substance 3, EBV positive control substance 4, VZV positive control substance 5, negative control substance 6, instructions 7 and box body 8 form.
[0029] The quantitative PCR reaction solution contains PCR buffer, MgCl 2 , dNTPs, thermostable DNA polymerase, EBV upstream amplification primers, EBV downstream amplification primers, VZV upstream amplification primers, VZV downstream amplification primers, EBV fluorescent probes and VZV fluorescent probes.
[0030] The primer sequences for PCR amplification are:
[0031] EBV upstream amplification primer sequence (SEQ ID No: 1): 5'-TCATCTACGGGGACACGGAC-3';
[0032]EBV downstream amplification primer sequence (SEQ ID No: 2): 5'-GAGCTCCACCCCCTTCATC-3';
[0033] VZV upstream amplification primer sequence (SEQ ID No: 3): 5'-TAGGCGGATGG...
Embodiment 2
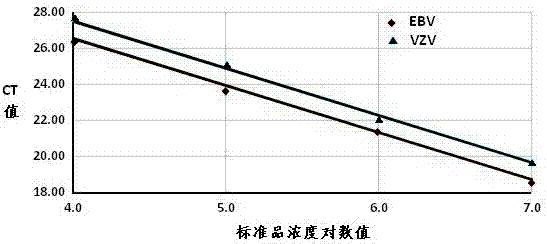
[0041] Example 2 Sensitivity and specificity test of human herpesvirus EBV and VZV detection kits
[0042] (1) Materials:
[0043] The selected pathogenic microorganisms include: experimental group: EBV (strain B95-8) provided by Beijing Institute of Virology, VZV (strain EF) provided by the microbiology teaching and research group of Anhui Medical University; control group: Staphylococcus aureus, Escherichia coli, type B Hepatitis virus, Cryptococcus neoformans, Candida albicans and human genome were provided by the Bacteria Room, Virus Room and Gene Amplification Room of Children's Hospital Affiliated to Zhejiang University.
[0044] (2) Design and synthesis of primers and probes:
[0045] Conduct bioinformatics analysis on the conserved region sequences of EBV and VZV, design and screen PCR amplification primers and specific fluorescent probes, and entrust Shanghai Sangon Biotech Co., Ltd. to synthesize them.
[0046] EBV upstream amplification primer sequence (SEQ ID...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com