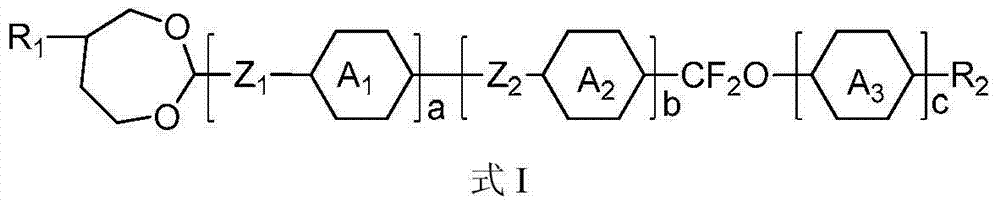
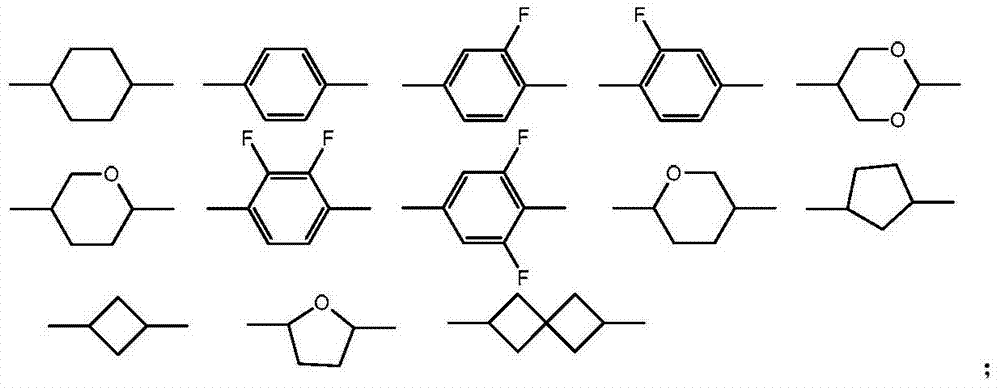
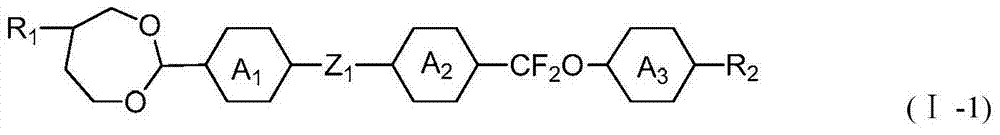
2-methyl-1,3-dioxepane derivative
A technology of alkyl and compound, applied in the field of liquid crystal compound synthesis and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1, Synthesis of Compound I-17 (Synthesis Route 1)
[0112]
[0113] step 1:
[0114]
[0115] Add 44.4g (0.24mol) p-bromobenzaldehyde (reactant), 21.6g (0.24mol) (reactant) of 1,4-butanediol, 400ml dry toluene (solvent), 2g p-toluenesulfonic acid to a 1L three-necked flask , heated to reflux for 4 hours, separated the formed water, cooled to room temperature, washed with 100ml of water, and evaporated to dryness to obtain 55.5g of (I-17-a), with a yield of 90% and a purity of 98% by gas chromatography.
[0116] step 2
[0117] Add 25.7g (0.1mol) (I-17-a) (reactant) and 150ml tetrahydrofuran (solvent) into the reaction flask, protect it with nitrogen, cool down to -60°C, add dropwise 0.2mol n-butyllithium (reactant ) of petroleum ether (solvent) solution, the dropwise addition was completed within 1 hour, and the reaction was stirred at -50°C for 30 minutes. Then cool down to -60°C, add dropwise 13g (0.13mol) trimethyl borate (reactant) in 70ml tetrahy...
Embodiment 2
[0130] Example 2, Synthesis of Compound I-18 (Synthesis Route 2)
[0131] step 1
[0132]
[0133] Add 0.1mol (Ⅰ-17-a), 0.12mol m-fluorophenylboronic acid (reactant), 0.13mol sodium carbonate (reactant), 80ml toluene (solvent), 60ml ethanol (solvent), 60ml water (solvent) to the reaction bottle ), under the protection of nitrogen, add 0.4g tetrakis (triphenylphosphine) palladium (catalyst), stir and heat to reflux for 3 hours. Cool down to room temperature, separate the layers, extract the aqueous phase with 50ml of toluene (solvent), and wash the organic phase with water until neutral. Evaporate the solvent to dryness, dissolve the resultant in 100ml of toluene, decolorize it through a silica gel column, elute with toluene (solvent), collect the eluent and evaporate the solvent to dryness, dissolve with 3 times of petroleum ether, freeze and recrystallize at -20°C, and filter with suction. 24.5 g of (I-18-a) were obtained as white crystals. The yield is 90%, and the gas...
Embodiment 3
[0149] Embodiment 3, the synthesis of compound I-19
[0150] step 1
[0151]
[0152] Add 25.7g (0.1mol) (Ⅰ-17-a) (reactant) and 120ml tetrahydrofuran (solvent) to the reaction bottle, install the seal and stir, replace the air with nitrogen, drop the temperature to -70°C, and add 0.1mol Concentration of 2.5M butyllithium (reactant), 20 minutes after the addition, dry carbon dioxide gas (reactant), to saturation, react at this temperature for 2 hours, pour this reaction solution into 20ml concentrated Hydrochloric acid (to adjust the pH value) and 100ml of water in a beaker for hydrolysis, liquid separation, 50ml of ethyl acetate (solvent) to extract the water phase once, combine the organic phase, wash with saturated saline until neutral, dry with anhydrous sodium sulfate (drying agent), Concentrate to remove the solvent to obtain a light yellow solid, which is recrystallized once with 2 times of toluene and 1 times of ethyl acetate (solvent) to obtain 20 g of white cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com