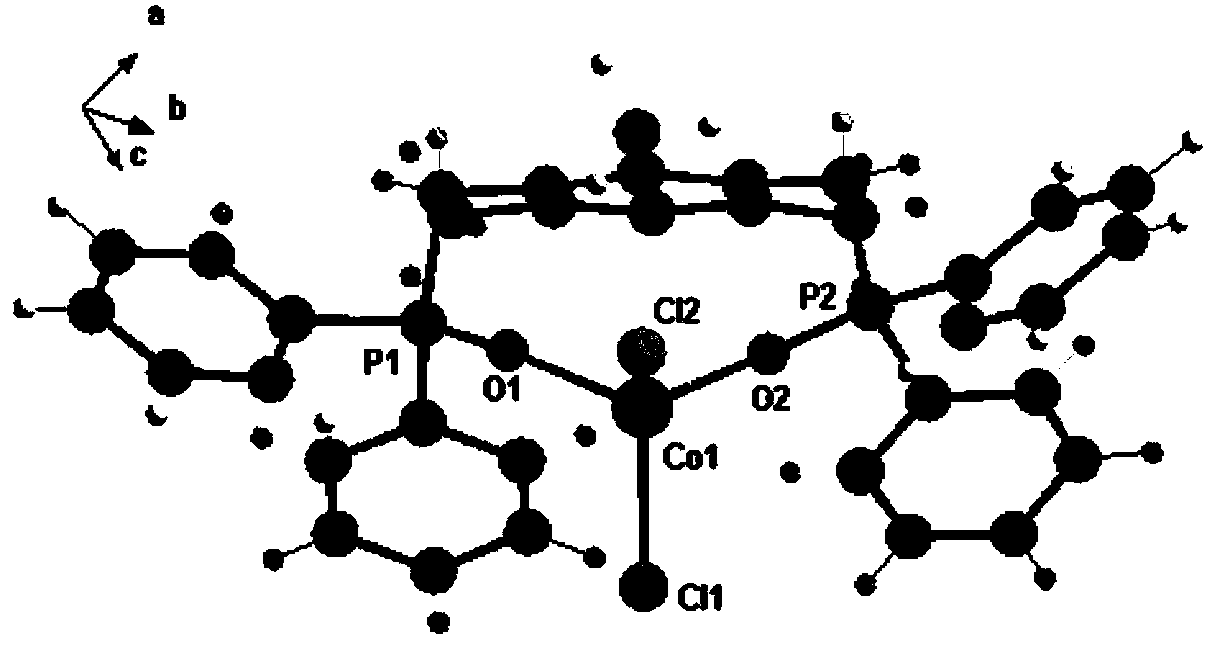
Pincer-shaped skeleton ligand containing phosphine bond and preparation of ligand, and preparation method of Pincer-shaped organic compound
A pincer-shaped, skeleton-like technology, applied in the field of preparation of Pincer-type metal-organic compounds, to achieve the effects of high yield, low cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
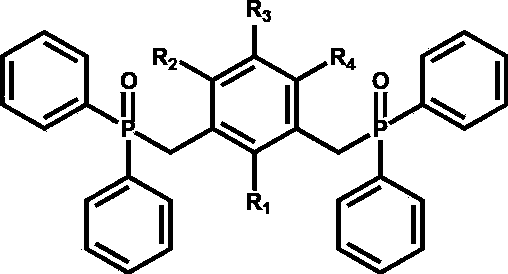
[0022] In the structural formula of the pincer skeleton ligand containing a phosphono bond, when R1, R2 and R4 are hydrogen, R3 is a methyl group.
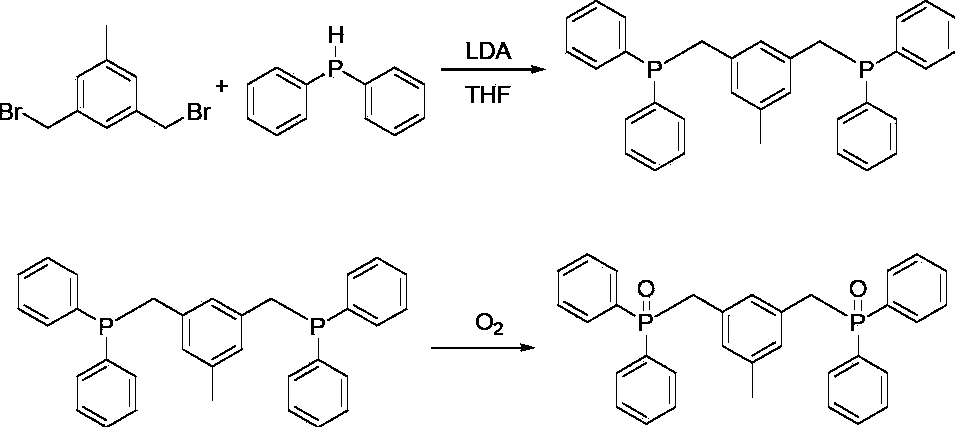
[0023] The synthetic route of this phosphono bond-containing pincer skeleton ligand is as follows:
[0024]
[0025] The synthesis method is:
[0026] (1) Dissolve 8 mL of diphenylphosphine in 60 mL of tetrahydrofuran, cool down to -78°C, add 26 mL of cyclohexane containing 0.52 mol of lithium diisopropylamide into the above tetrahydrofuran containing diphenylphosphine, and stir for reaction 2 -3h. Then rise to room temperature and react for 30 min.
[0027] (2) Cool down the reaction solution obtained in step (1) to -78°C, and drop 100mL of tetrahydrofuran containing 0.018mol 3,5-dibromomethyltoluene into the reaction solution obtained in step (1) in the above step (1) when the temperature is lowered to -78°C , and dropwise addition was completed within 30 minutes. The reaction was then stirred overnight at room temperatur...
Embodiment 2
[0032] When R1, R2 and R4 are methyl groups in the structural formula of the pincer skeleton ligand containing phosphono bonds, R3 is hydrogen.
[0033] The synthetic route of this phosphono bond-containing pincer skeleton ligand is as follows:
[0034]
[0035] The synthesis method is:
[0036] (1) Mix m-trimethylbenzene and paraformaldehyde at a molar ratio of 1:2, dissolve in 40 mL of acetic acid solution containing 33% hydrobromic acid by mass ratio, and react at 80°C for 8 hours. Then add water, stir and analyze for 1-2h, filter and wash, and dry to obtain a white solid. The solid was recrystallized in chloroform to give 2,4-dibromomethyl-1,3,5-trimethylbenzene.
[0037] (2) Dissolve 12.5mL of diphenylphosphine in 125mL of tetrahydrofuran, lower to -78°C, then add 40mL of cyclohexane containing 0.2mol of lithium diisopropylamide into the tetrahydrofuran of diphenylphosphine, and stir Reaction 2-3h. Then rise to room temperature and react for 30min.
[0038] (3) Lo...
Embodiment 3
[0043] The difference from Example 1 is that the 1,3-dibromobenzyl derivative can be prepared from 1,5-dibromomethyl-2,4-dimethylbenzene, 1,5-dibromomethyl-2, 3,4-trimethylbenzene or 1,3-dibromomethyl-2,4,5,6-tetramethylbenzene for replacement; the specific preparation is to mix benzene derivatives and paraformaldehyde at a molar ratio of 1:2 Mix and add 40-50mL of hydrobromic acid in 33% acetic acid solution, react at 80°C for 6-12 hours; add water after reaction, stir for 1-2 hours, filter and wash, and dry to obtain a white solid in chloroform Recrystallized and set aside.
[0044] The benzene derivatives are toluene, m-trimethylbenzene, m-xylene, 1,2,3-trimethylbenzene or 3,4,5-trimethyltoluene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com