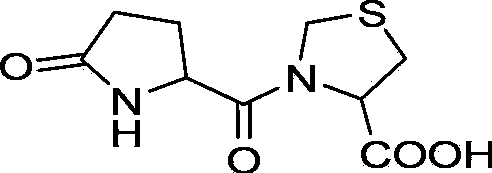
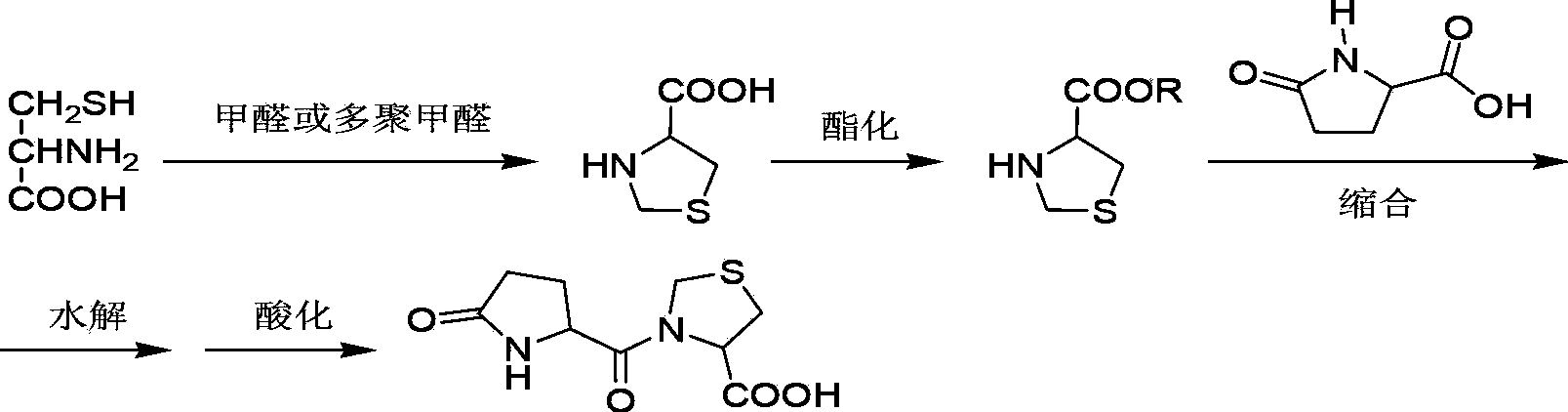
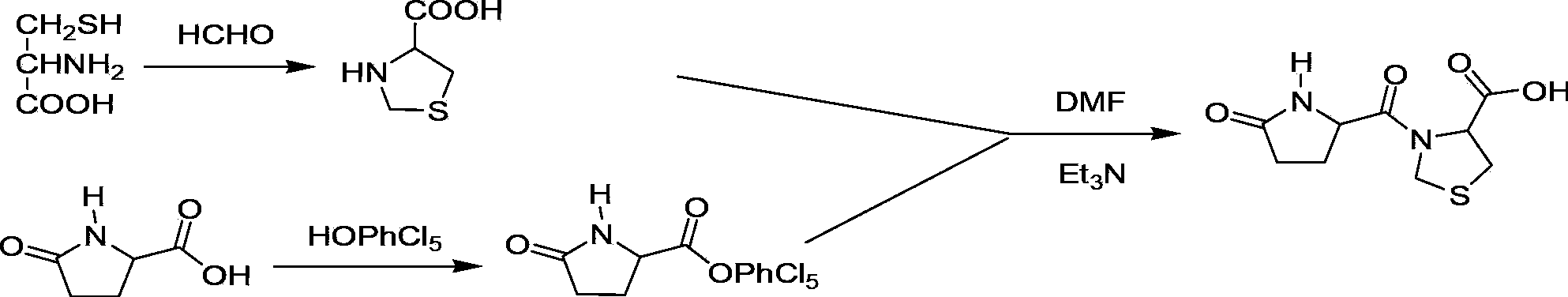
Preparation method of pidotimod
A technology based on pidotimod and time, which is applied in the field of pharmaceutical synthesis, can solve the problems of low solubility of pidotimod, high toxicity of reagent pentachlorophenol, unstable active ester, etc., so as to reduce production cost, low cost, The effect of easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 pidotimod
[0040] In a dry 2000mL three-necked round-bottom flask, add 50.0g (0.375mol) of L-pyroglutamic acid and 700ml of anhydrous tetrahydrofuran, stir, cool to -5~5°C, add 39.7g (0.375mol) of sodium carbonate, Add 40.7 g (0.375 mol) of ethyl chloroformate dropwise under temperature control -5 to 5°C. After the dropwise addition is completed, keep stirring and react for 20 minutes, add 49.9 g (0.375 mol) of L-thiazolidinyl-4-carboxylic acid, and continue to control Stir and react at -5~5°C for 20 minutes, raise the reaction temperature to room temperature, evaporate the reaction solution to dryness under reduced pressure, add 200ml of 50% ethanol to the residue, stir to dissolve it completely, and control the temperature at -20~-10 30ml of concentrated hydrochloric acid was added dropwise at ℃, after the dropwise addition was completed, the crystallization was carried out with stirring and heat preservation for 8 hours, filtered, and...
Embodiment 2
[0043] The preparation of embodiment 2 pidotimod
[0044] In a dry 2000mL three-neck round bottom flask, add 50.0g (0.375mol) of L-pyroglutamic acid and 700ml of anhydrous tetrahydrofuran, stir, cool down to -20~-10°C, add 39.7g (0.375mol) of sodium carbonate , add 40.7g (0.375mol) of ethyl chloroformate dropwise under temperature control at -20~-10°C, after the dropwise addition is complete, keep stirring for 10 minutes, then add 49.9g (0.375mol) of L-thiazolidinyl-4-carboxylic acid, Continue to control the temperature at -5 ~ 5 ° C and stir for 20 minutes, then raise the reaction temperature to room temperature, evaporate the reaction solution to dryness under reduced pressure, add 200 ml of 60% ethanol to the residue, stir to dissolve it completely, and control the temperature at -20 ~ Add 30ml of concentrated hydrochloric acid dropwise at -10°C, after the dropwise addition is completed, keep stirring and crystallize for 20h, filter, and dry to obtain 71.8g of crude pidotim...
Embodiment 3
[0047] The preparation of embodiment 3 pidotimod
[0048] In a dry 2000mL three-neck round bottom flask, add 50.0g (0.375mol) of L-pyroglutamic acid and 700ml of anhydrous tetrahydrofuran, stir, cool down to -20~-10℃, add 24.3g (0.412mol) of trimethylamine , add 40.7g (0.412mol) of ethyl chloroformate dropwise under temperature control at -20~-10°C, after the dropwise addition is completed, keep stirring for 10 minutes, then add 49.9g (0.375mol) of L-thiazolidinyl-4-carboxylic acid, Continue to control the temperature at -5 ~ 5 °C and stir for 20 minutes, then raise the reaction temperature to room temperature, evaporate the reaction solution to dryness under reduced pressure, add 200 ml of 30% ethanol to the residue, stir to dissolve it completely, and control the temperature at -20 ~ Add 30ml of concentrated hydrochloric acid dropwise at -10°C. After the dropwise addition, keep stirring and crystallize for 16h, filter, and dry to obtain 72.9g of crude pidotimod, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com