Glycosylated derivative of cyclic peptide compounds and preparation method and application thereof
A technology of glycosylation and derivatives, which is applied in the field of glycosylation derivatives of cyclic peptide compounds and its preparation, can solve the problems of limited application due to drug resistance, large side effects, narrow antibacterial spectrum, etc., and achieve improved antifungal activity , strong inhibitory activity, broad-spectrum antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 High-resolution mass spectrum and proton nuclear magnetic spectrum data of compounds 1a-1g of the present invention
[0030] See Table 1 and Table 2 for the high-resolution mass spectrum and proton NMR data of compounds 1a-f of the present invention.
[0031] Table 1 High resolution mass spectrum table of compound 1a-f of the present invention
[0032]
[0033] Table 2 Compounds of the present invention 1a-f The H NMR Data Sheet
[0034]
[0035]
Embodiment 2
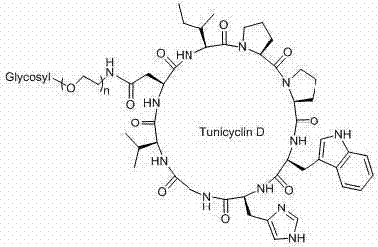
[0036] Example 2 Compounds of the invention 1a-g preparation of
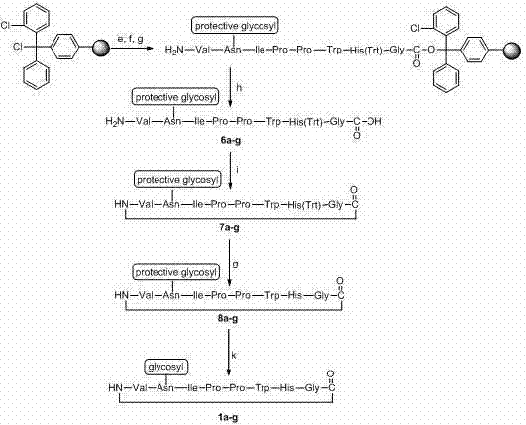
[0037] 2.1 Compounds of the present invention 1a-f The preparation can be roughly divided into two steps:
[0038] 2.1.1 Preparation of key intermediate glycosyl activated ester 5a-g , the reaction scheme is as follows:
[0039]
[0040] 2.1.2 Preparation of target compounds 1a-g , the reaction scheme is as follows:
[0041]
[0042] 2.2 Compounds of the present invention 1a - g The specific preparation method is as follows:
[0043] 2.2.1 Preparation of azide side chains
[0044] Weigh sodium azide (45 g, 0.69 mol) into a 250 mL three-neck flask, add 2-chloroethanol (32 mL, 0.58 mol), and add catalyst tetrabutylammonium bromide (TBAB, 4 g ), heated to reflux for 12 h, the reaction solution changed from a khaki suspension to a brown suspension. Lift to cool, add 60 mL of anhydrous ether, filter with suction, wash the filter cake with 30 mL of anhydrous ether, and combine the filtrates. S...
Embodiment 3
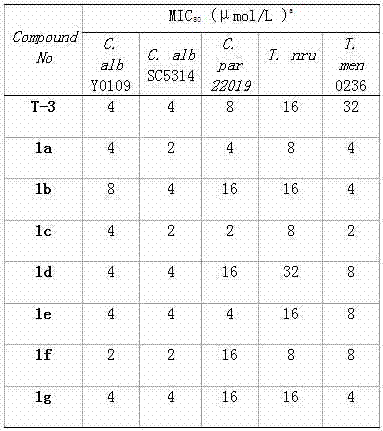
[0057] Example 3 Pharmacological experiments of the compounds of the present invention
[0058] 3.1 Experimental method: The micro liquid base dilution method recommended by the CLSI-M27A3 and M38-A2 documents of the Clinical and Laboratory Standards Institute (CLSI) was adopted.
[0059] 3.1.1 Experimental strains
[0060] In this experiment, the following 6 kinds of 7 common human pathogenic standard fungal strains were selected as the screening objects:
[0061] Two ATCC standard strains:
[0062] Candida albicans SC5314
[0063] Cryptococcus neoformans 32609
[0064] Five clinical strains:
[0065] Candida glabrata (Candida krusei) 537
[0066] Candida parapsilosis 22019
[0067] Trichophyton rubrum Cmccftla
[0068] Microsporum gypseum Cmccfmza
[0069] Candida albicans Y0109
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com