Preparation method for benzoic acid
A technology of benzoic acid and benzophenone, which is applied in the field of preparation of benzoic acid, can solve the problems of backward development of organic photochemistry, etc., and achieve the effects of reducing the cost of the reaction process, improving cleanliness, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
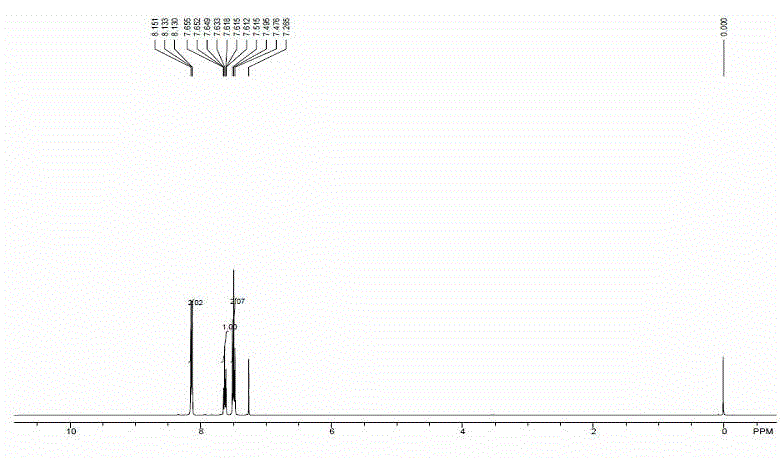
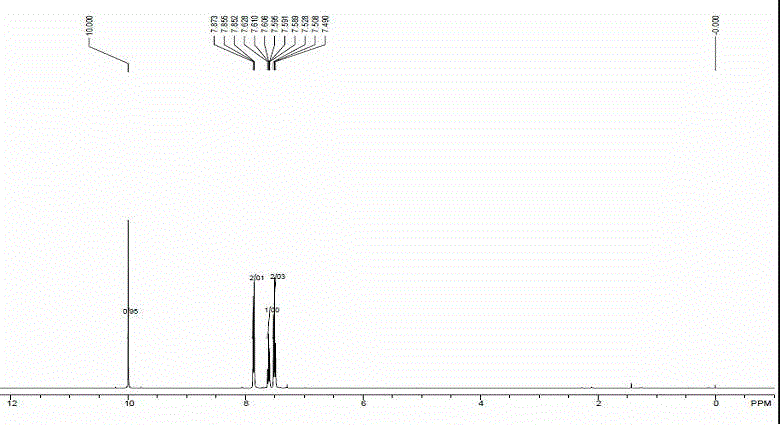
[0022] In a 50 mL quartz reaction vial, add 92 mg toluene, 10 mL solvent acetonitrile, 5 mg benzophenone, magnetically stir in air atmosphere, at 25 o C temperature, Xe lamp (300 W) under light for 10 hours. After the reaction time was up, only a single product, benzoic acid, was generated through high-performance liquid chromatography analysis. Rotary evaporation in vacuum, separation and purification by column chromatography, the product quality is 51 mg, and the yield rate is 42%, [ 1 H NMR (400 MHz, CDCl 3 ): δ 12.26 (brs, 1 H), 8.13 (d, J = 8.0 Hz, 2 H), 7.65-7.55 (m, 1 H), 7.54-7.30 (m, 2 H)]. Keeping other conditions unchanged, change the temperature of the above reaction, respectively at 0 o C, 50 o Carried out at C temperature, temperature increase or decrease all make the productive rate of this reaction reduce.
Embodiment 2
[0026] In a 50 mL quartz reaction flask, add 92 mg toluene, 10 mL solvent acetonitrile, 10 mg benzophenone, magnetically stir in the air atmosphere, at 25 o C temperature, under the light of Xe lamp (300 W) for 6 hours. After the reaction time was up, only a single product, benzoic acid, was generated through high-performance liquid chromatography analysis. Rotary evaporation in vacuum, separation and purification by column chromatography, the product quality is 82 mg, and the yield is 67%. Keeping other conditions unchanged, change the temperature of the above reaction, respectively at 0 o C, 50 o Carried out at C temperature, temperature increase or decrease all make the productive rate of this reaction reduce.
Embodiment 3
[0028] In a 50 mL quartz reaction vial, add 92 mg toluene, 10 mL solvent acetonitrile, 20 mg benzophenone, magnetically stir in air atmosphere, at 25 o C temperature, under the light of Xe lamp (300 W) for 3 hours. After the reaction time was up, only a single product, benzoic acid, was generated through high-performance liquid chromatography analysis. Rotary evaporation in vacuum, separation and purification by column chromatography, the product quality is 120 mg, and the yield is 98%. Keeping other conditions unchanged, change the temperature of the above reaction, respectively at 0 o C, 50 o Carried out at C temperature, temperature increase or decrease all make the productive rate of this reaction reduce.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com