A common repaglinide tablet and preparation method thereof
A common tablet and filler technology, applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low dissolution rate, high impurity content and poor stability of repaglinide tablets and other problems, to achieve the effect of weak hydrophobicity, simple process and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A common repaglinide tablet, comprising the following raw materials in parts by weight:
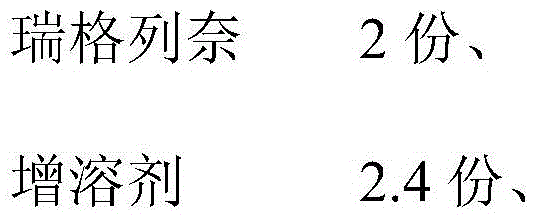
[0028]
[0029]
[0030] Among them, the solubilizer is meglumine, the binder is hypromellose, the disintegrant is carboxymethylcellulose calcium, the lubricant is sodium stearyl fumarate, the filler is 55 parts of mannitol, corn starch 25 parts, 75 parts of calcium hydrogen phosphate, 18 parts of microcrystalline cellulose.
[0031] A preparation method of repaglinide common sheet, comprising the steps of:
[0032] Step 1: pulverize repaglinide, sieve, and weigh the prescription amount for later use;
[0033] Step 2: Weighing the solubilizer, binder, disintegrating agent and filler of the prescribed amount, mixing evenly, and preparing auxiliary materials for use;
[0034] Step 3: mix the excipients prepared in step 2 with the repaglinide prepared in step 1 according to the principle of equal increase, add the prescribed amount of lubricant, and mix evenly;
[0035] Step ...
Embodiment 2
[0037] A common repaglinide tablet, comprising the following raw materials in parts by weight:
[0038]
[0039]
[0040] Wherein, the solubilizer is meglumine, the binder is povidone K30, the disintegrant is carboxymethylcellulose calcium, and the lubricant is sodium stearyl fumarate.
[0041] A preparation method of repaglinide common sheet, comprising the steps of:
[0042] Step 1: pulverize repaglinide, sieve, and weigh the prescription amount for later use;
[0043] Step 2: Weighing the solubilizer, binder, disintegrating agent and filler of the prescribed amount, mixing evenly, and preparing auxiliary materials for use;
[0044] Step 3: mix the excipients prepared in step 2 with the repaglinide prepared in step 1 according to the principle of equal increase, add the prescribed amount of lubricant, and mix evenly;
[0045] Step 4: Test the intermediate content, calculate the tablet weight according to the specifications and intermediate content data, and use the s...
Embodiment 3
[0047] A common repaglinide tablet, comprising the following raw materials in parts by weight:
[0048]
[0049]
[0050] Wherein, the solubilizer is meglumine, the binder is povidone K30, the disintegrant is carboxymethylcellulose calcium, and the lubricant is sodium stearyl fumarate.
[0051] A preparation method of repaglinide common sheet, comprising the steps of:
[0052] Step 1: pulverize repaglinide, sieve, and weigh the prescription amount for later use;
[0053] Step 2: Weighing the solubilizer, binder, disintegrating agent and filler of the prescribed amount, mixing evenly, and preparing auxiliary materials for use;
[0054] Step 3: mix the excipients prepared in step 2 with the repaglinide prepared in step 1 according to the principle of equal increase, add the prescribed amount of lubricant, and mix evenly;
[0055] Step 4: Test the intermediate content, calculate the tablet weight according to the specifications and intermediate content data, and use the sem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com