Preparation process of rear earth yttrium ion doped tetracalcium phosphate
A tetracalcium phosphate, preparation technology, applied in the direction of phosphorus compounds, inorganic chemistry, non-metallic elements, etc., can solve the problems of secondary sintering, lower TTCP quality, increase process difficulty and production cost, etc., to reduce process difficulty and Effects of production cost, lower temperature, and increased ion diffusion speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Calcium hydrogen phosphate and analytically pure calcium carbonate were mixed in a molar ratio of 1:2, ball milled on a ball mill with water as a medium for 6 hours, then dried in a drying oven at 80°C for 2 hours, and then the mixture was moved to a high-temperature furnace at 250 The heating rate of ℃ / h is raised to 1380 ℃, and after holding for 4 hours, the sample is directly taken out of the furnace and quenched to obtain tetracalcium phosphate.
Embodiment 2
[0012] Calcium hydrogen phosphate and analytically pure calcium carbonate were mixed in a molar ratio of 1:2, ball milled on a ball mill with water as a medium for 6 hours, then dried in a drying oven at 80°C for 2 hours, and then the mixture was moved to a high-temperature furnace at 250 The heating rate of ℃ / h is raised to 1380 ℃, and after holding for 4 hours, the tetracalcium phosphate is produced in the furnace and cooled with the furnace.
Embodiment 3
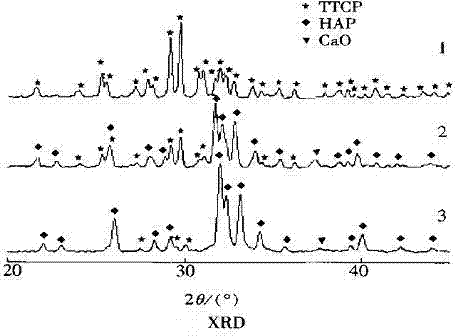
[0014] Calcium hydrogen phosphate and analytically pure calcium carbonate were mixed according to a molar ratio of 1:2, and yttrium nitrate with a total mass of calcium hydrogen phosphate and analytically pure calcium carbonate of 1.2% was added and ball milled on a ball mill for 6 hours with water as a medium, and then placed in a drying oven at Dry at 80°C for 2 hours, then move the mixture to a high-temperature furnace to raise the temperature to 1380°C at a rate of 250°C / h, keep it warm for 4 hours, and then cool down with the furnace in the furnace to prepare rare earth yttrium ion-doped tetracalcium phosphate.
[0015] The XRD analysis results of the product calcined at 1380°C for 4 hours showed that in the TTCP sample doped with 1.2wt% yttrium nitrate, the main crystal phase was TTCP, and the diffraction peaks of calcium oxide (CaO) and HA were hardly observed, while no yttrium nitrate doped The diffraction peaks of calcium oxide (CaO) and HA appeared obviously in the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com