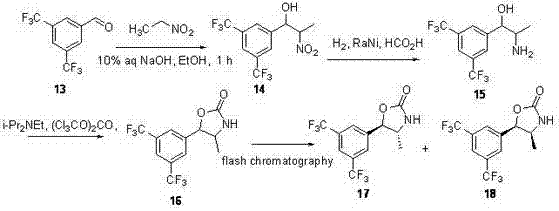
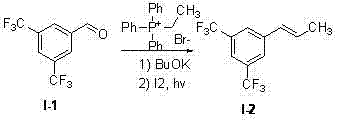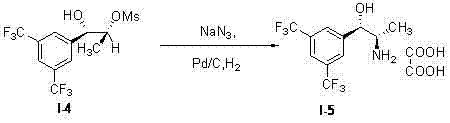Synthesis method of key intermediate of anacetrapib
A technology of anseltrapib and synthetic methods, applied in the direction of organic chemistry, etc., can solve problems such as difficult to meet cholesterol ester transfer protein, and achieve the effect of improving drug purity, high selectivity, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Step a:
[0050]
[0051] Ph 3 Preparation of PEtBr
[0052] Triphenylphosphine (200g, 0.76mol), dissolved in toluene (600mL), added bromoethane (200mL, 2.66mol), then stirred and reacted at 70°C for 48 hours, the resulting solid was washed with petroleum ether, and dried in vacuo to obtain a white Solid (240g, 85%);
[0053] Preparation of I-2
[0054] Weigh the above-prepared triphenylethylphosphine bromide (74.25g, 0.20mol) and dissolve it in 375mL of anhydrous tetrahydrofuran, cool to 0°C, add potassium tert-butoxide (24g, 0.21mol) to avoid violent exotherm. Inverted at room temperature and stirred for 0.5 hours to obtain a dark red solution. Add 250 mL of anhydrous THF solution containing 3,5-bis(trifluoromethyl)benzaldehyde (I-1) (36.3 g, 0.15 mol) dropwise under stirring. 40 minutes. Under cooling, the reaction was quenched with 200 mL of water until the reaction did not exotherm violently. Extracted with 500 mL×3 ethyl acetate, combined the organic lay...
Embodiment 2
[0084] Step a:
[0085]
[0086] Ph 3 Preparation of PEtBr
[0087] Triphenylphosphine (150g, 0.57mol), dissolved in toluene (450mL), added bromoethane (150mL, 2.0mol), then stirred and reacted at 70°C for 48 hours, the resulting solid was washed with petroleum ether, and dried under vacuum to obtain a white Solid (200g, 94%);
[0088] Preparation of I-2
[0089] Weigh the above-prepared triphenylethylphosphine bromide (37.1g, 0.10mol) and dissolve it in 200mL of anhydrous tetrahydrofuran, cool to 0°C, add potassium tert-butoxide (13.5g, 0.12mol) to avoid violent exotherm . Inverted at room temperature and stirred for 50 minutes to obtain a dark red solution. Add dropwise 121 mL of anhydrous THF solution containing 3,5-bis(trifluoromethyl)benzaldehyde (I-1) (24.2 g, 0.10 mol) under stirring. 30 minutes. Under cooling, the reaction was quenched with 150 mL of water until the reaction was not exothermic violently. Extract with 200 mL×3 ethyl acetate, combine the organ...
Embodiment 3
[0114] Step a:
[0115]
[0116] Ph 3 Preparation of PEtBr
[0117] Triphenylphosphine (300g, 1.14mol), dissolved in toluene (900mL), added bromoethane (300mL, 4.0mol), then stirred and reacted at 70°C for 48 hours, the resulting solid was washed with petroleum ether, and dried in vacuo to obtain a white Solid (370g, 87%);
[0118] Preparation of I-2
[0119] Weigh the above-prepared triphenylethylphosphine bromide (74.25g, 0.20mol) and dissolve it in 1100mL of anhydrous tetrahydrofuran, cool to 5°C, add potassium tert-butoxide (29g, 0.26mol) to avoid violent exotherm. Inverted at room temperature and stirred for 1.0 hour to obtain a dark red solution. Add dropwise 322 mL of anhydrous tetrahydrofuran solution containing 3,5-bis(trifluoromethyl)benzaldehyde (I-1) (32.2 g, 0.133 mol) under stirring. 60 minutes. Under cooling, the reaction was quenched with 200 mL of water until the reaction did not exotherm violently. Extracted with 500 mL×3 ethyl acetate, combined the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com