Small-molecule inhibitor of Mdm<X>/Mdm<2>, as well as preparation method and applications
A technology of small molecule inhibitors and chemical structural formulas, which is applied in the field of medicinal chemistry technology and can solve the problems of no cis-imidazoline framework, no reversible inhibitors, and no such problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: A preparation method of a small molecule inhibitor of MdmX / Mdm2:
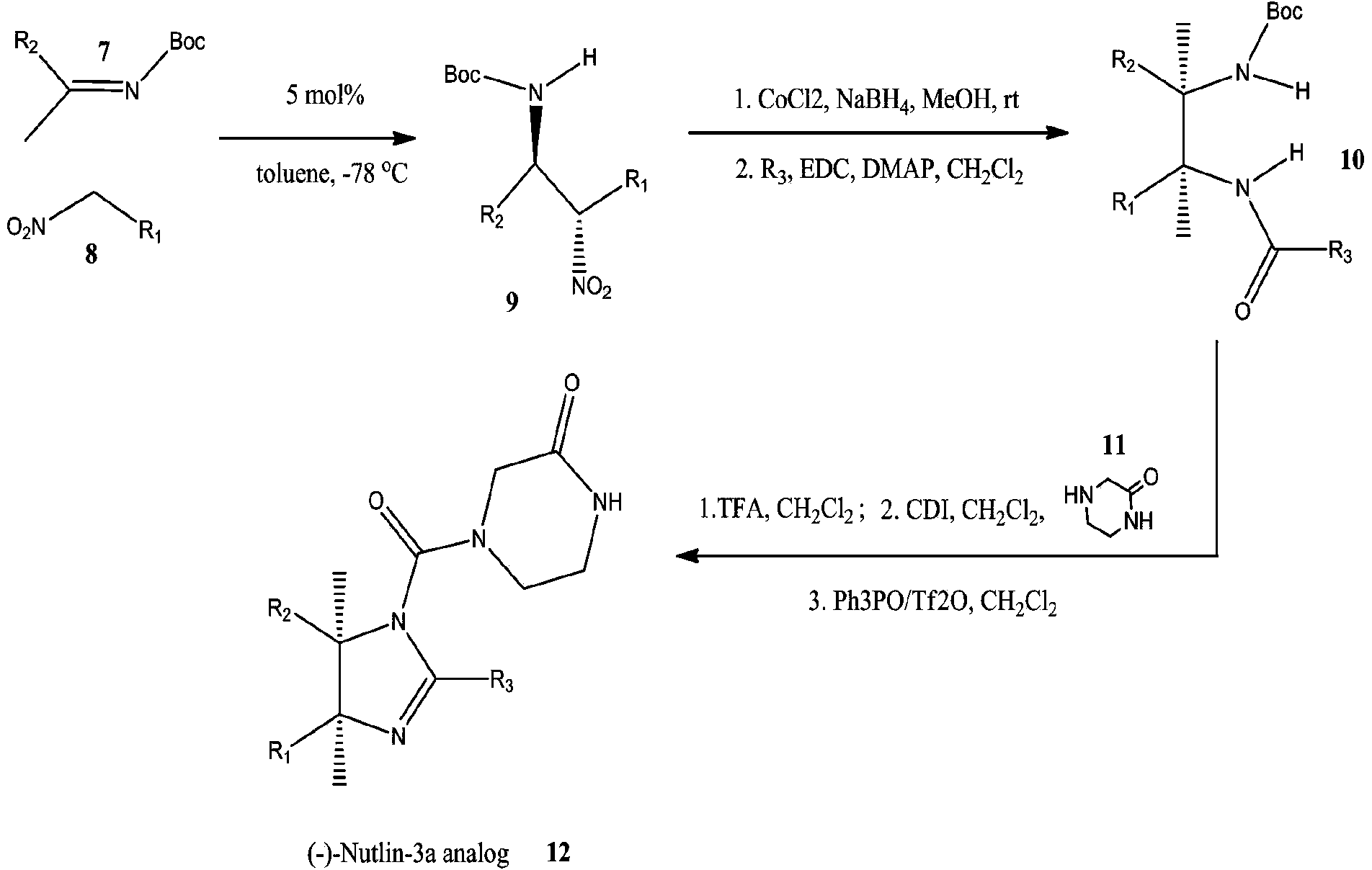
[0057] The small molecule inhibitors of MdmX / Mdm2 are cis-imidazolines, and their synthesis routes are as follows: figure 1 As shown, the principle is to use a diastereoselective and stereoselective catalyst to catalyze the addition reaction of arylnitromethane and Schiff base (Davis, T.A. & Johnston, J.N. Chem. Sci.2, 1076-1079, 2011).
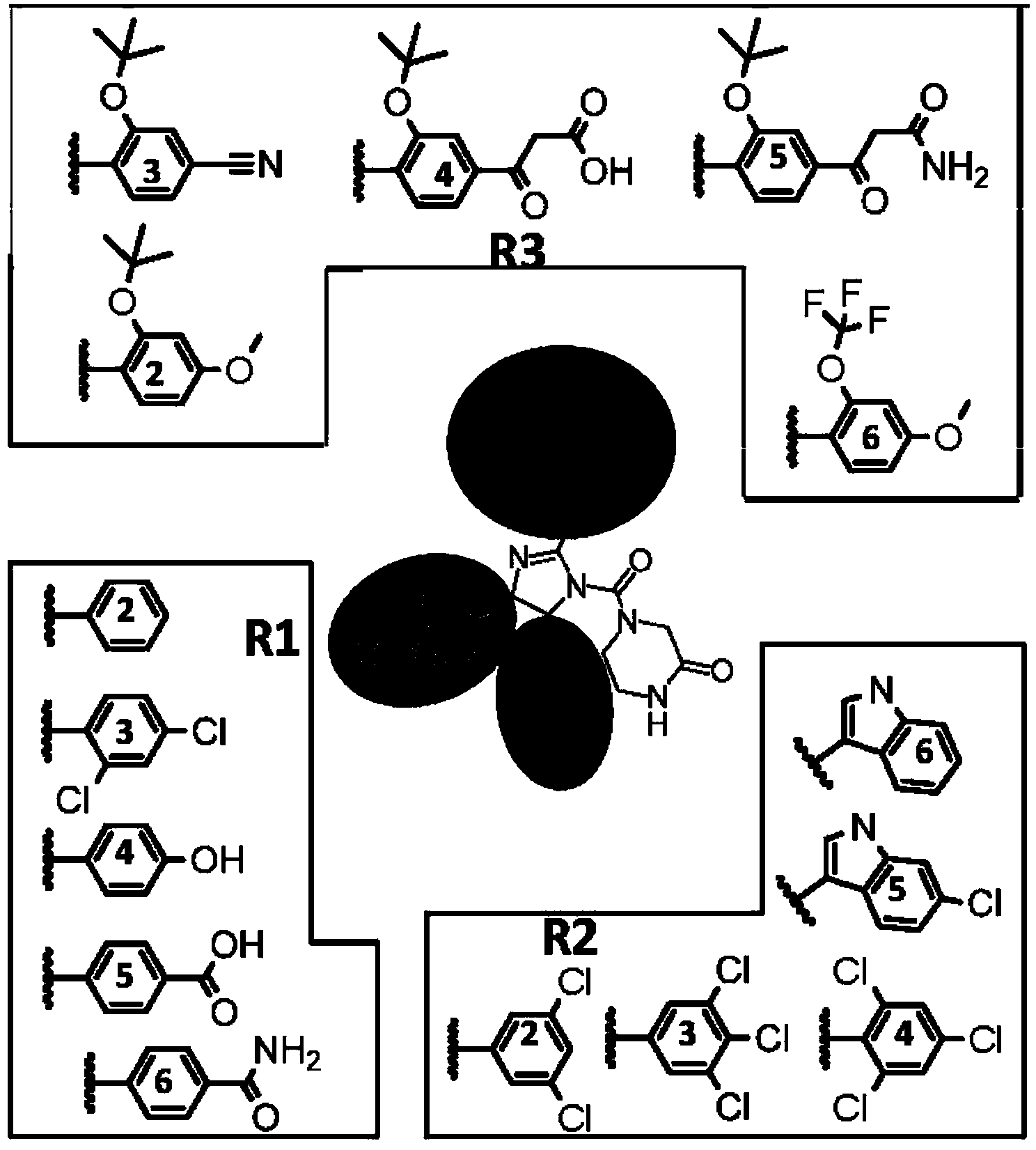
[0058] R 1 The functional group was introduced using nitrobenzene (compound 8). Using halogenated benzene as raw material, prepared according to the method described by Kornblum (JACS,1956,78:1497), functional group R 1 (4) Hydroxyl, R 1 Carboxyl and R in (5) 1 The amides in (6) are respectively protected by methyl ether, methyl ester and benzyl, and deprotected by hydrolysis in the later stage of synthesis.
[0059] R 2 The functional group was introduced using aryl imine (compound 7). Using α-aminophenyl sulfone or α-aminoindole sulfone as raw materi...
Embodiment 2
[0063] Embodiment 2: MdmX and Mdm2 protein sample preparation method:
[0064] The amino-terminal amino acid 22-110 sequence fragment of human MdmX (N-MdmX) and the Mdm2 amino-terminal amino acid 22-110 (N-Mdm2) sequence fragment were synthesized according to the codon preference of Escherichia coli. The artificially synthesized DNA fragments were respectively used Restriction endonucleases BamH I and EcoR I were treated for use.
[0065] The commercialized pET28a plasmid (Novagen) was used for protein expression, and its original thrombin cleavage site (Leu-Val-Pro-Arg-Gly-Ser) was replaced with the TEV protease cleavage site (Glu -Asn-Leu-Tyr-Phe-Gln-Gly), the new plasmid was named pSD. pSD was treated with the same restriction endonuclease for use.
[0066] The N-Mdm2 and MdmX DNA fragments treated with BamH I and EcoR I were respectively connected to the pSD vector treated with the same restriction enzymes to construct the N-Mdm2 and MdmX protein expression plasmids, pSD...
Embodiment 3
[0070] Embodiment 3, high-throughput MdmX inhibitor screening method:
[0071] MdmX inhibitor screening was determined by fluorescence polarization (FP) method. Performed in assay buffer containing 10 mM Tris (pH 8.0), 200 mM NaCl and 0.01% Tween-20. The human p53 protein amino acid 15-29 sequence fragment peptide (p53p) was labeled with fluorescein (fluorescein-GSGSSQETFSDLWKLLPEN, Flu-p53p). Mutant p53 peptides (fluorescein-GSGSSQETASDLAKLAPEN, Flu-p53pAAA) were used as negative controls.
[0072]FP detection was performed using 15nM fluorescein and 1μM N-MdmX in a buffer containing 10mM Tris (pH 8.0), 200mM NaCl and 0.01% Tween-20. In the N-MdmX / p53p inhibitor assay, the nutlin analog was premixed with the protein for 30 minutes, then the labeled peptide was added and mixed for 30 minutes. The (Corning) FP assay was then performed in 384-well black microplates. FP assay analysis was performed using an EnVision multilabel microplate reader with 555nm excitation filter, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com