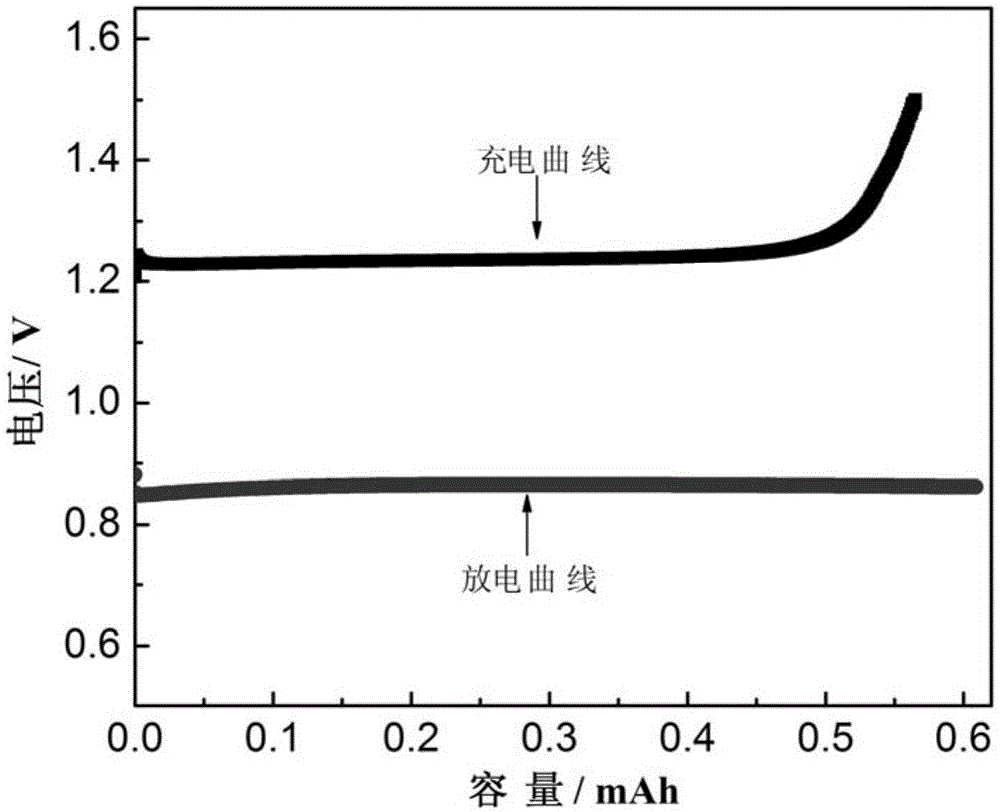
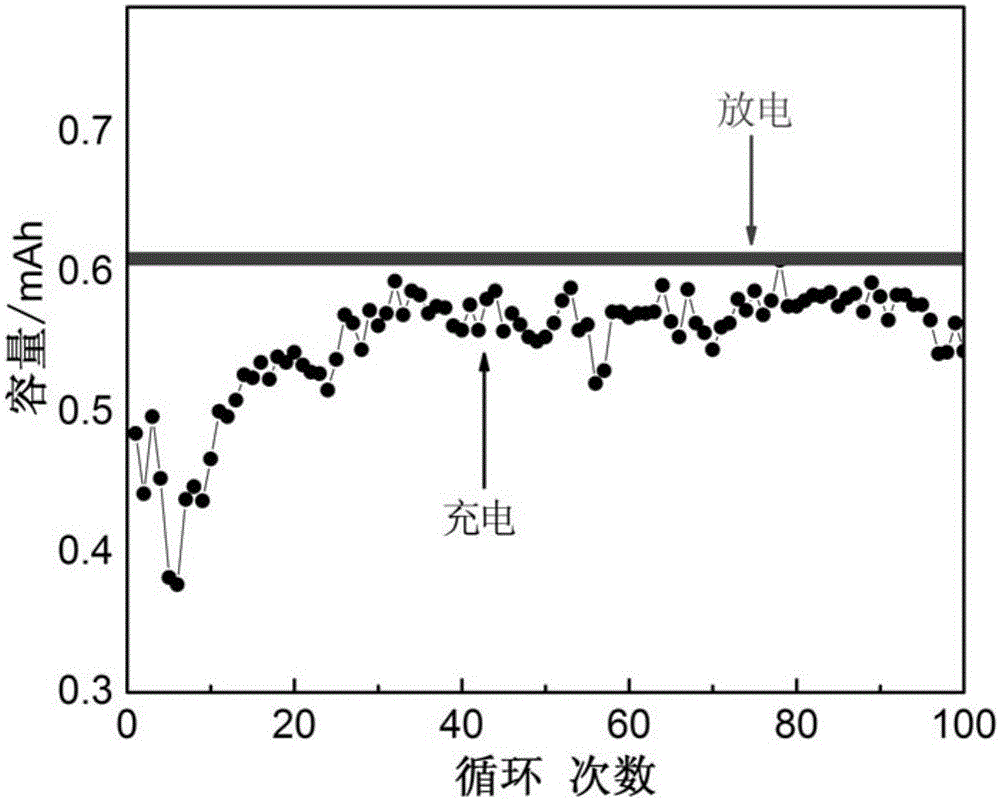
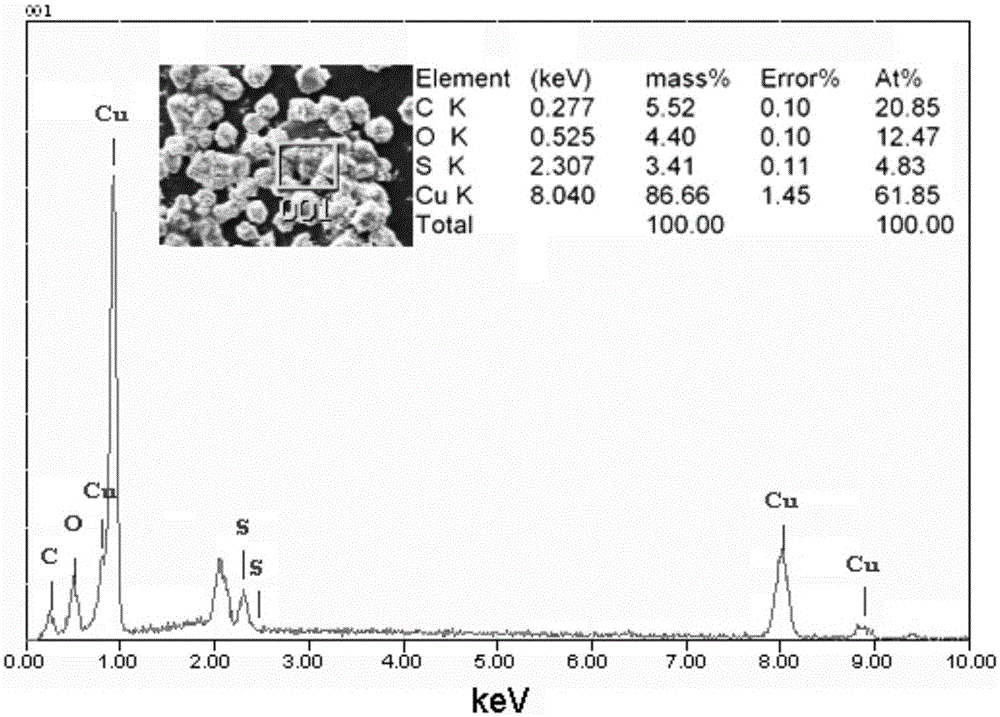
a rechargeable battery
A zinc battery, charging and discharging technology, applied in battery electrodes, alkaline storage batteries, secondary battery manufacturing, etc., can solve problems such as irreversibility of copper-zinc batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] This embodiment describes a kind of metal copper as the positive electrode current collector, copper sulfate solution as the positive electrode electrolyte, zinc sheet as the negative electrode current collector, zinc sulfate as the negative electrode electrolyte, lithium ions as the conductive ion, and NASICON type lithium titanium aluminum phosphate as the Copper-zinc reversible battery with separator.
[0016] 【1】Positive electrode: Prepare 15wt% CuSO 4 +15wt%Li 2 SO 4 An aqueous solution, a copper sheet with a thickness of 0.5 mm was immersed in the solution as a positive electrode.
[0017] [2] Negative electrode: Prepare 15wt% ZnSO 4 +15wt%Li 2 SO 4 An aqueous solution in which a zinc sheet with a thickness of 0.5mm is immersed in the solution as a negative electrode.
[0018] 【3】Diaphragm: Li with a thickness of 0.3mm 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 Ceramics.
[0019] The above-mentioned positive and negative electrodes and separators are assembled in an ...
Embodiment 2
[0021] This embodiment describes a carbon brush as the positive electrode collector, the mixed solution of copper acetate and copper chloride as the positive electrode electrolyte, the zinc sheet as the negative electrode collector, the mixed solution of zinc formate and zinc pinate as the negative electrode electrolyte, and the sodium ion Copper-zinc reversible battery with conductive ions and perovskite sintered ceramics as separator.
[0022] 【1】Positive electrode: Prepare 5wt% Cu(CH 3 COO) 2 +10wt%CuCl 2 +5wt%NaH 2 PO 4 An aqueous solution, the carbon brush is immersed in the solution as the positive electrode.
[0023] 【2】Negative electrode: Prepare 2wt% Zn(HCOO) 2 +10wt%Zn(NO 3 ) 2 +10wt%LiNO 3 Water-glycerol (volume ratio 4:1) solution, a zinc sheet with a thickness of 0.5 mm is immersed in the solution as a negative electrode.
[0024] 【3】Diaphragm: Perovskite ceramic sheet with a thickness of 0.3mm.
[0025] The above-mentioned positive and negative electrod...
Embodiment 3
[0027] This embodiment describes a kind of metal copper as positive electrode collector, copper oxalate and copper oxide mixed suspension as positive electrode electrolyte, stainless steel sheet as negative electrode collector, zinc citrate and lithium zincate solution as negative electrode electrolyte, lithium The ions are conductive ions, and the copper-zinc reversible battery is a Garnet-type ceramic as a diaphragm.
[0028] [1] Positive electrode: Mix copper oxalate and copper oxide in a molar ratio of 1:1, add propylene carbonate, dimethyl carbonate, ethyl methyl carbonate (volume ratio 1:1:1) mixed solvent in a ratio of 1:4 , add lithium hexafluorophosphate to make the concentration 1molL -1 , after adding 5wt% polymethyl methacrylate and ball milling, the copper sheet was immersed in the suspension as the positive electrode.
[0029] [2] Negative electrode: take zinc citrate and zinc hydroxide, mix them at a molar ratio of 1:5, add 10wt% lithium hydroxide aqueous solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com