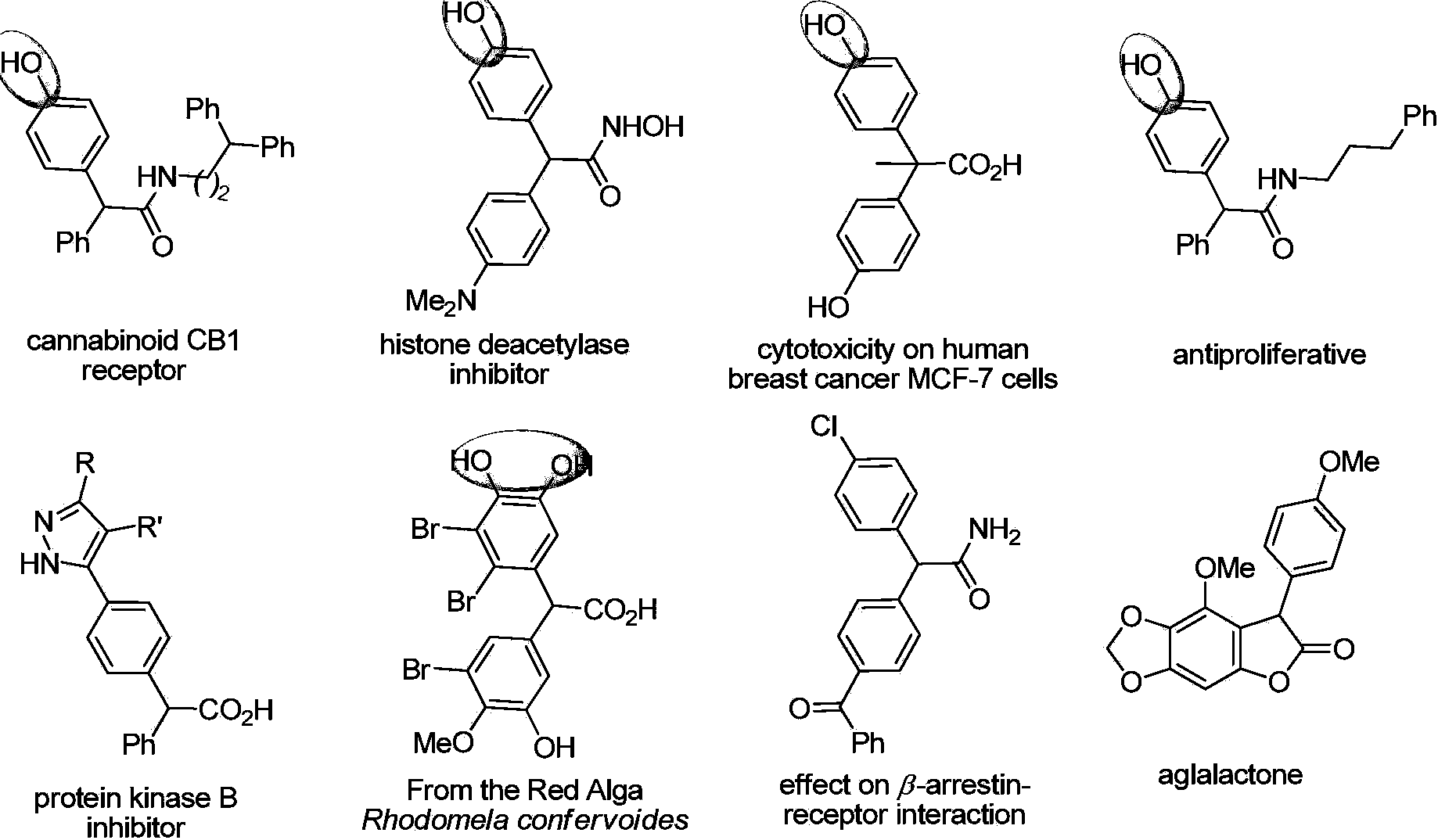
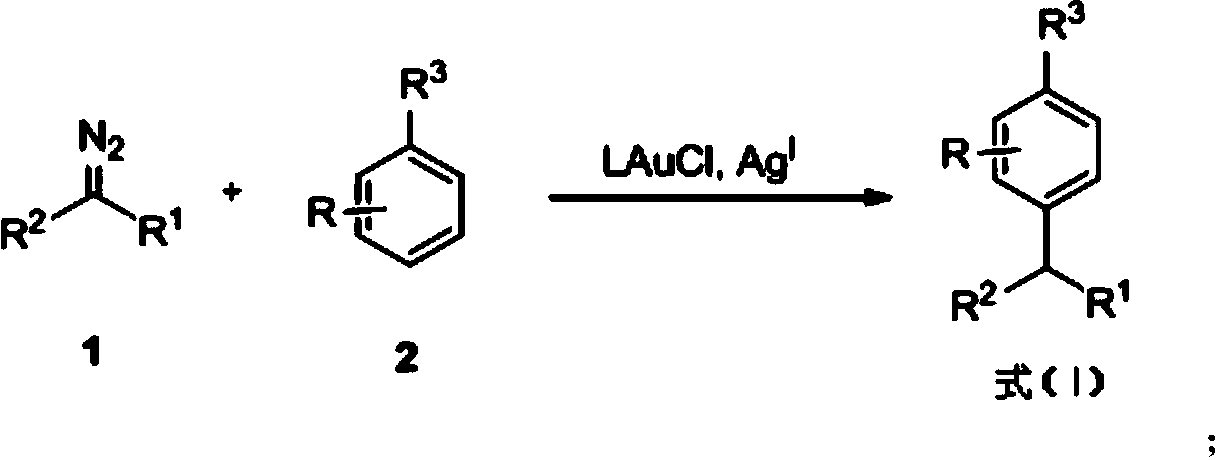
Method for performing gold-catalyzed selective C-H bond functionalization on phenol and aniline
A functionalized and selective technology, applied in the field of C-H bond functionalization with high chemical and regioselectivity, can solve problems such as poor chemical selectivity, achieve less catalyst dosage, maintain yield and catalytic efficiency, and reduce substrates. Wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Synthesis of 2-(4-hydroxyphenyl)-2-phenylacetic acid methyl ester (Methyl2-(4-hydroxyphenyl)-2-phenylacetate)
[0053]
[0054] Step 1: To a pre-dried reaction tube, add (2,4- t Bu 2 C 6 h 3 O) PAuCl (0.02mmol) and previously with CaH 2 Dried CH 2 Cl 2 (4mL), then add AgSbF 6 (0.02 mmol), stirred for 15 minutes. In the second step, phenol (0.6mmol) was first added to the reaction tube, and then the diazo compound (0.4mmol) was pre-dissolved in CaH 2 Dried CH 2 Cl 2 (1mL) then dropwise add the dichloromethane solution of the diazo compound, the whole dropping process continues for 30 minutes, the slower the dropping process, the better, especially at the end of the fast dropwise addition, so as to avoid the spontaneous generation of the diazo compound get together. The entire reaction process should be avoided under strong light conditions to avoid decomposition of the silver salt. After the dropwise addition was completed, stirring was continued...
Embodiment 2
[0055] Example 2 Synthesis of ethyl 2-(4-hydroxyphenyl)-2-phenylacetate (Ethyl2-(4-hydroxyphenyl)-2-phenylacetate).
[0056]
[0057] Operation reference example 1 yield is 99%. 1 H NMR (400MHz, CDCl 3 )δ7.20-7.35(m, 5H), 7.13(d, J=8.4Hz, 2H), 6.71(d, J=8.4Hz, 2H), 5.61(s, 1H), 4.95(s, 1H), 4.20(q, J=7.2Hz, 2H), 1.24(t, J=7.2Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ173.30154.88, 138.86, 130.58, 129.78, 128.55, 128.41, 127.15, 115.44, 61.36, 56.27, 14.06; MS (EI): m / z (%): 256 (M + , 10.47); 183(100); HRMScalcd.for C 16 h 16 o 3 : HRMS (ESI) calcd.for C 16 h 16 NaO 3 [M+Na]: 279.0992, found: 279.0981.
Embodiment 3
[0058] Example 3 Synthesis of 2-(4-hydroxyphenyl)-2-(4-chlorophenyl)methyl acetate (Methyl2-(4-chlorophenyl)-2-(4-hydroxyphenyl)acetate).
[0059]
[0060] Operation reference example 1 yield is 99%. 1 H NMR (400MHz, CDCl 3 )δ7.28(d, J=8.0Hz, 2H), 7.22(d, J=8.0Hz, 2H), 7.13(d, J=8.0Hz, 2H), 6.76(d, J=8.0Hz, 2H) , 5.13(s, 1H), 4.94(s, 1H), 3.74(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ173.10, 154.97, 137.30, 133.18, 130.26, 129.84, 129.73, 128.71, 115.57, 55.46, 52.49; MS (EI): m / z (%): 276 (M + , 29.32), 278([M+2] + , 9.13); 217(100); HRMS(ESI) calcd.for C 15 h 13 ClNaO 3 [M+Na]: 299.0445, found: 299.0449.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com