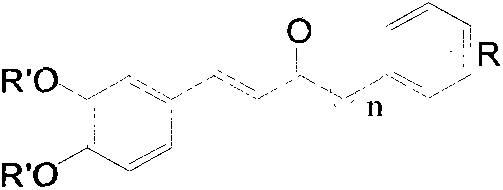
Preparation method of E-3,4-dihydroxyphenylvinyl ketone and application thereof as nerve protection drug
The technology of a dihydroxyphenyl and compound is applied in the preparation of E-3,4-dihydroxystyryl ketone compounds and its application field as a neuroprotective drug, which can solve the problem of difficulty in permeating the blood-brain barrier, metabolic rate wait for the question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
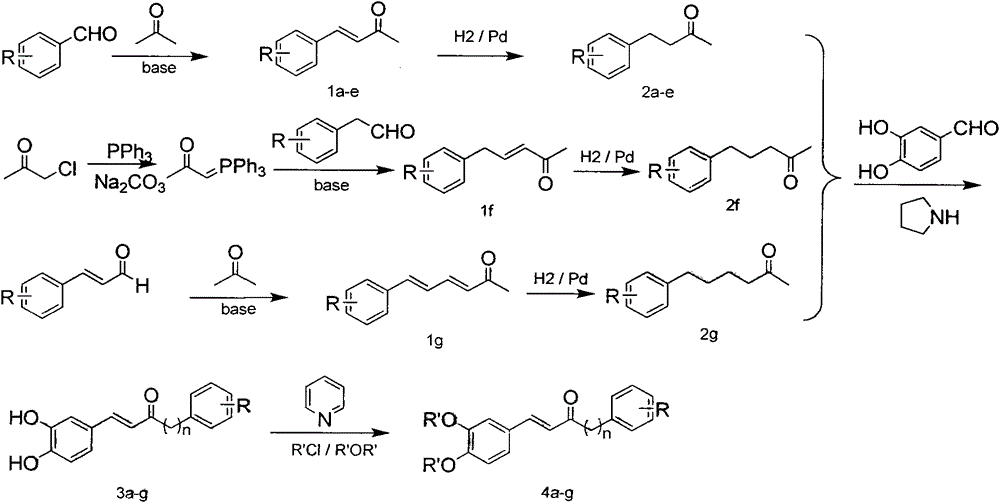
[0028] Preparation of 1-phenylbut-1-en-3-one (compound 1a)
[0029] After adding 5ml of acetone to 5ml of 10% NaOH aqueous solution, slowly add benzaldehyde (5.30g, 0.05mol) in acetone solution (10ml) dropwise, control the reaction temperature not to exceed 30°C, drop it for about 1h, continue the reaction for 3h, and detect by TLC The response is complete. The reaction solution was neutralized to PH=7 with 10% HCl, the solvent was spin-dried under reduced pressure, extracted with ethyl acetate, the ester layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was spin-dried under reduced pressure, separated and purified by silica gel column (eluted with petroleum ether / ethyl acetate) to obtain the target compound 1a, 6.0 g of light yellow crystals, and the yield was 82.5%. m.p.39-41°C. 1 HNMR (400MHz, CDCl 3 )δ: 7.46 (d, .J=16.2Hz, 1H, C2-H), 7.14-7.30 (m, 5H, Ar-H), 6.61 (d, J=16.2Hz, 1H, C1-H), 2.30 (s, 3H, CH 3 )
[003...
Embodiment 2
[0043] Preparation of 4-phenylbutan-2-one (compound 2a)
[0044] Dissolve 1a (0.29g, 2.0mmol) in dichloromethane (10ml), add 10% Pb / C (30mg), react in hydrogen atmosphere for 12h, TLC detects that the reaction is complete, filter with celite, and decompress the filtrate After spin-drying, separation and purification on a silica gel column (elution with petroleum ether / ethyl acetate) gave the target compound 2a as a colorless liquid with a yield of 90.0%.
[0045] Preparation of 4-(3-chlorophenyl)-butan-2-one (2b)
[0046] Using the synthesis method of compound 2a above, 2b was obtained by using 1-(3-chlorophenyl)-but-1-en-3-one as a reactant with a yield of 89.3%.
[0047] Preparation of 4-(3,4-dihydroxyphenyl)-butan-2-one (2c)
[0048] Using the synthesis method of compound 2a above, 2c was obtained by using 1-(3,4-dihydroxyphenyl)-but-1-en-3-one as a reactant, with a yield of 85.7%, m.p.85-86°C.
[0049] Preparation of 4-(3-methoxy-4-hydroxyphenyl)-butan-2-one (2d)
[00...
Embodiment 3
[0058] Preparation of E-1-(3,4-dihydroxyphenyl)-5-phenyl-pent-1-en-3-one (3a)
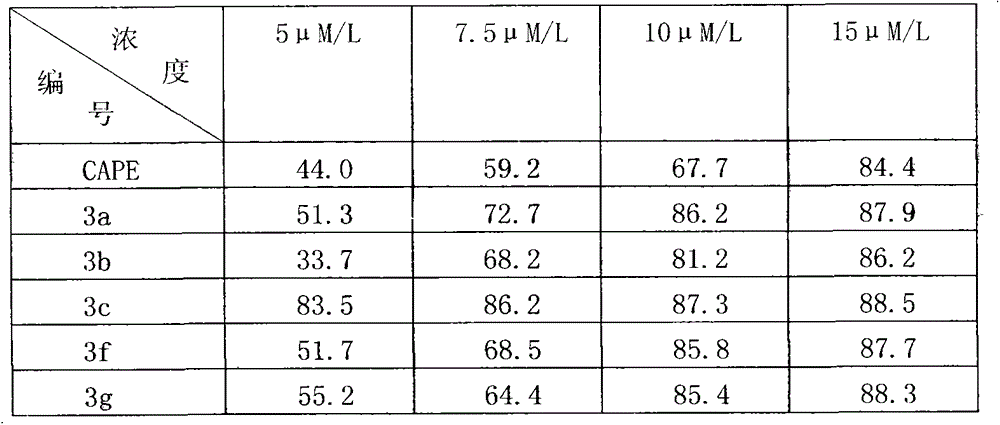
[0059] Add 4-phenylbutan-2-one (0.45g, 3mmol) and a catalytic amount of pyrrolidine and acetic acid into tetrahydrofuran (30ml) solvent and stir, slowly add 3,4-dihydroxybenzaldehyde (0.38g, 3mmol) of tetrahydrofuran (20ml) solution, drop it for 1h, stir the reaction at room temperature in the dark, TLC detects that the reaction is complete, spin down the solvent under reduced pressure, add a small amount of water, extract with ethyl acetate, combine the ester layers, and wash with saturated saline Wash with saturated sodium bisulfite solution, dry over anhydrous sodium sulfate, spin dry the solvent under reduced pressure, separate and purify with silica gel column (petroleum ether / ethyl acetate elution) to obtain the target compound 3a as a yellow solid, 0.37g, yield 55.5% , m.p.153-155°C. 1 HNMR (400MHz, DMSO-d 6 )δ: 9.37(s, 2H, 2×OH), 7.49(d, J=16.2Hz, 1H, C2-H), 6.75-7.84(m, 8H, Ar-H), 6.53(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com