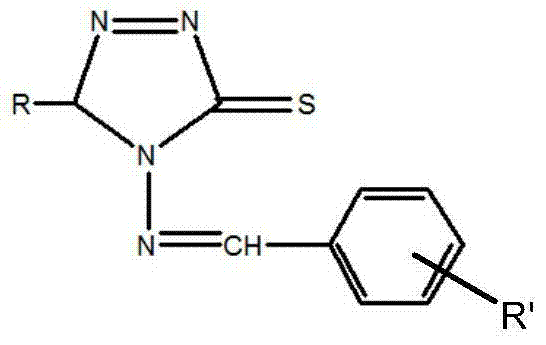
4-amino-5-substituted-1,2,4-triazole-3-thione Schiff base and preparation method thereof
An amino, thione technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield, large solvent usage, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
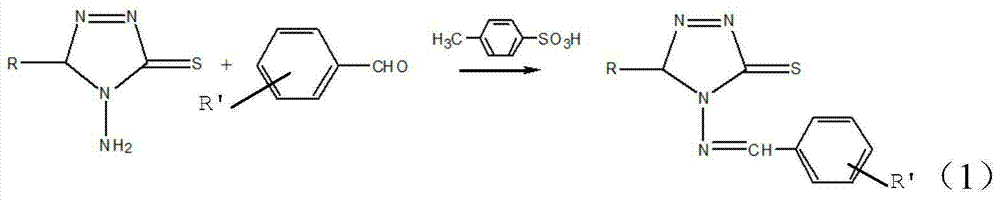
[0028] Wherein the preparation method of 4-amino-5-substituted-1,2,4-triazole-3-thione as raw material comprises the following steps:
[0029] 1) Add A mol carboxylic acid, B mol symmetrical thiosemicarbazide and C mol phosphorus pentachloride to a dry mortar, grind at room temperature for 5-10 minutes until the raw materials are completely reacted, and the crude product is obtained after standing; where A: B:C=(1~1.2):1:(1~1.2);
[0030] 2) Add an alkaline solution to the crude product until the pH of the resulting mixture is neutral, then suction-filter the mixture and recrystallize the filter cake to obtain 4-amino-5-substituted-1,2, 4-Triazole-3-thione.
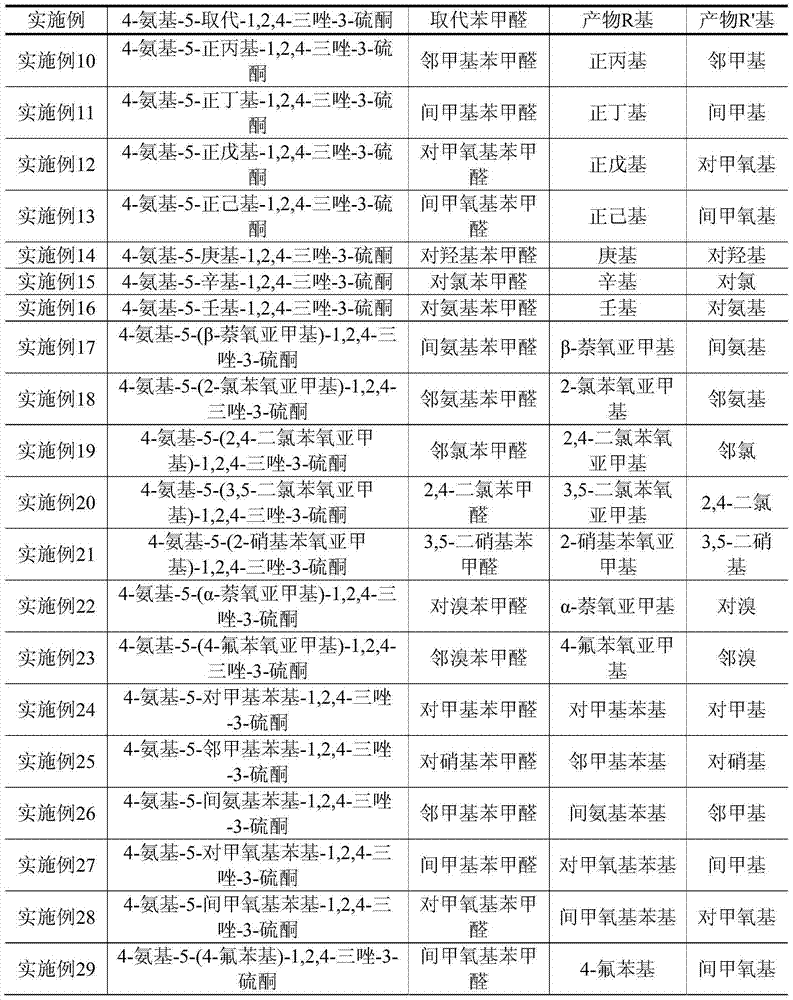
[0031]The carboxylic acid includes benzoic acid, p-toluic acid, o-toluic acid, m-toluic acid, p-methoxybenzoic acid, m-methoxybenzoic acid, 4-fluorobenzoic acid, 2-fluoro Benzoic acid, p-aminobenzoic acid, m-aminobenzoic acid, anthranilic acid, p-chlorobenzoic acid, o-chlorobenzoic acid, 2,4-dichlorobenzoic acid, p-nitr...
Embodiment 1
[0034] 1) Add 0.005mol 4-amino-5-phenyl-1,2,4-triazole-3-thione, 0.0055mol benzaldehyde and 0.0055mol p-toluenesulfonic acid to a dry mortar, and grind for 10min at room temperature, At this time, TLC monitoring showed that the raw material point of 4-amino-5-phenyl-1,2,4-triazole-3-thione disappeared, indicating that the raw material was completely reacted, and then left to stand for 30min to obtain the mixture; wherein the developer of TLC It is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0035] 2) Dissolve the mixture in a small amount of absolute ethanol, vacuum filter, wash the filter cake twice with a small amount of absolute ethanol, mix the eluate from the two times with the filtrate and concentrate to dryness to obtain high-purity 4-amino- 5-Phenyl-1,2,4-triazole-3-thione Schiff base whose R' group is -H. m.p.: 169-171°C, yield 87%.
[0036] IR (KBr tablet): 3172(N-H), 2990(=C-H, Ar-H), 1600(C=N), 1595, 1540, 1489, 1451(Ar), 13...
Embodiment 2
[0038] 1) Add 0.005mol 4-amino-5-p-chlorophenyl-1,2,4-triazole-3-thione, 0.0055mol benzaldehyde and 0.0055mol p-toluenesulfonic acid to a dry mortar and grind at room temperature 10min, at this time, TLC monitoring showed that the raw material point of 4-amino-5-p-chlorophenyl-1,2,4-triazole-3-thione disappeared, indicating that the raw material was completely reacted, and then left to stand for 30min to obtain a mixture; The developer of TLC is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0039] 2) Dissolve the mixture in a small amount of absolute ethanol, vacuum filter, wash the filter cake twice with a small amount of absolute ethanol, mix the eluate from the two times with the filtrate and concentrate to dryness to obtain high-purity 4-amino- 5-p-Chlorophenyl-1,2,4-triazole-3-thione Schiff base, the R' group is -H. m.p.: 172-174°C, yield 86.7%.
[0040] IR (KBr tablet): 3169(N-H), 2980(=C-H, Ar-H), 1591(C=N), 1580, 1529, 1489, 1452(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com