Phosphorescent fluorinion probe, preparation and applications thereof
A fluoride ion and probe technology, applied in the field of chemical/biosensors, can solve the problems of reducing detection sensitivity and signal-to-noise ratio, and achieve the effect of good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1, iridium complex, when Ligand is Time:
[0033] Compound 1: Preparation of 4-(Benzothiazol-2-yl)phenol
[0034]
[0035] Preparation of 4-(benzothiazol-2-yl)phenol: Weigh 2-aminobenzenethiol (4g) and p-hydroxybenzaldehyde (4.12ml), add them to a two-necked bottle, and vacuumize on the double-row tube- Nitrogen filling-vacuumizing, cycle three times, and finally protect the reaction system with nitrogen. Add pure N,N dimethylformamide (5ml) into the reaction flask, stir, and raise the temperature of the reaction system to 110°C, and the reaction time is about 72 hours. Treatment: Extract with water and ethyl acetate, spin dry in vacuo, and recrystallize from ethanol. Yield: 90%. 1 H NMR (400MHz,DMSO)δ10.21(s,1H),8.07(d,J=7.9Hz,1H),8.00–7.87(m,3H),7.53–7.45(m,1H),7.43–7.33( m,1H),6.97–6.88(m,2H).
[0036] Compound 2: Preparation of C^N Ligand
[0037]
[0038] Preparation of C^N ligand: Weigh 4-(benzothiazol-2-yl)phenol (1g)...
Embodiment 2
[0048] Embodiment 2, the preparation of iridium complex, when Ligand is Time:
[0049] Compound 1: Preparation of 4-(Benzothiazol-2-yl)phenol
[0050]
[0051] Preparation of 4-(benzothiazol-2-yl)phenol: Weigh 2-aminobenzenethiol (4g) and p-hydroxybenzaldehyde (4.12ml), add them to a two-necked bottle, and vacuumize on the double-row tube- Nitrogen filling-vacuumizing, cycle three times, and finally protect the reaction system with nitrogen. Add pure N,N dimethylformamide (5ml) into the reaction flask, stir, and raise the temperature of the reaction system to 110°C, and the reaction time is about 72 hours. Treatment: Extract with water and ethyl acetate, spin dry in vacuo, and recrystallize from ethanol. Yield: 90%. 1 H NMR (400MHz,DMSO)δ10.21(s,1H),8.07(d,J=7.9Hz,1H),8.00–7.87(m,3H),7.53–7.45(m,1H),7.43–7.33( m,1H),6.97–6.88(m,2H).
[0052] Compound 2: Preparation of C^N Ligand
[0053]
[0054] Preparation of C^N ligand: Weigh 4-(benzothiazol-2-yl)phenol (1g)...
Embodiment 3
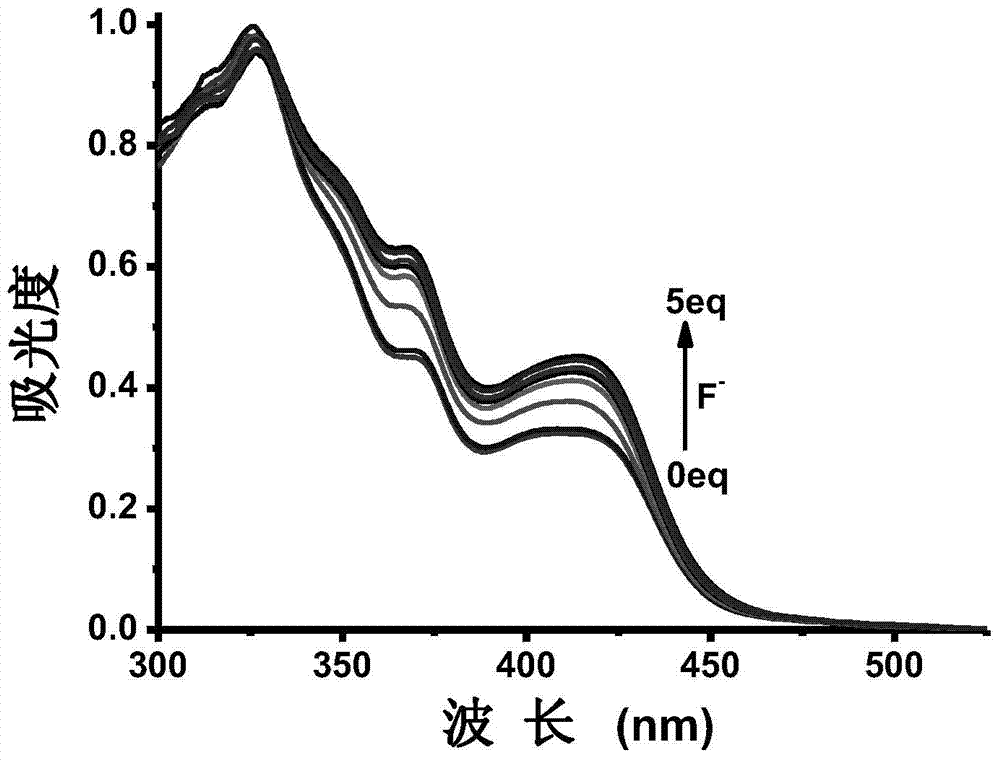
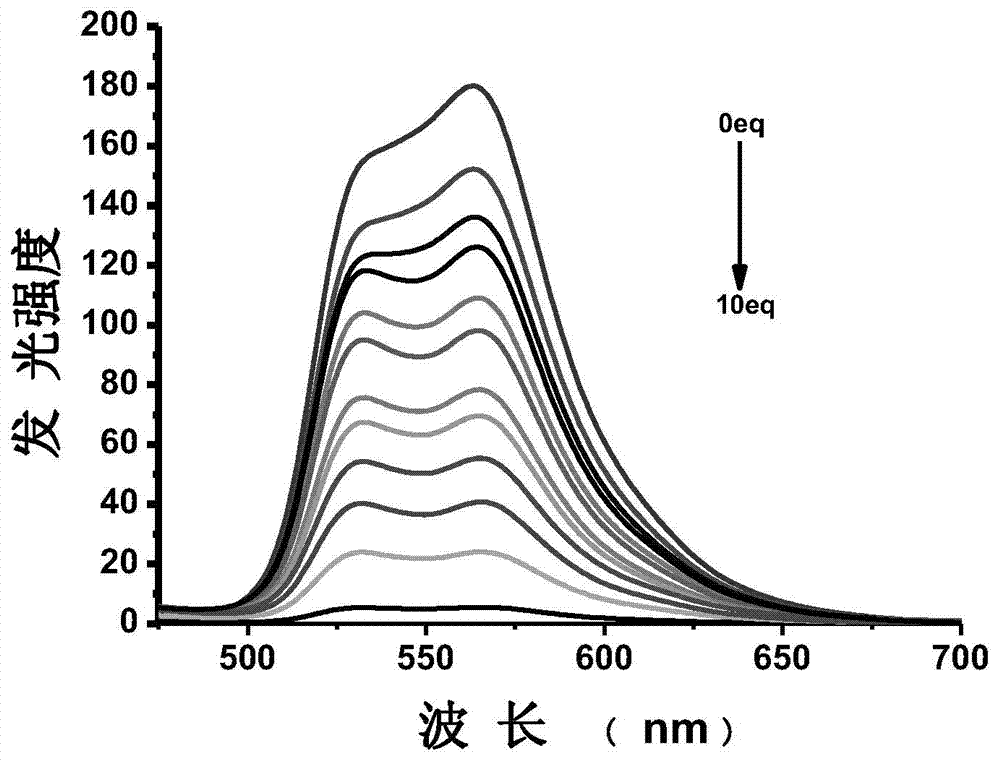
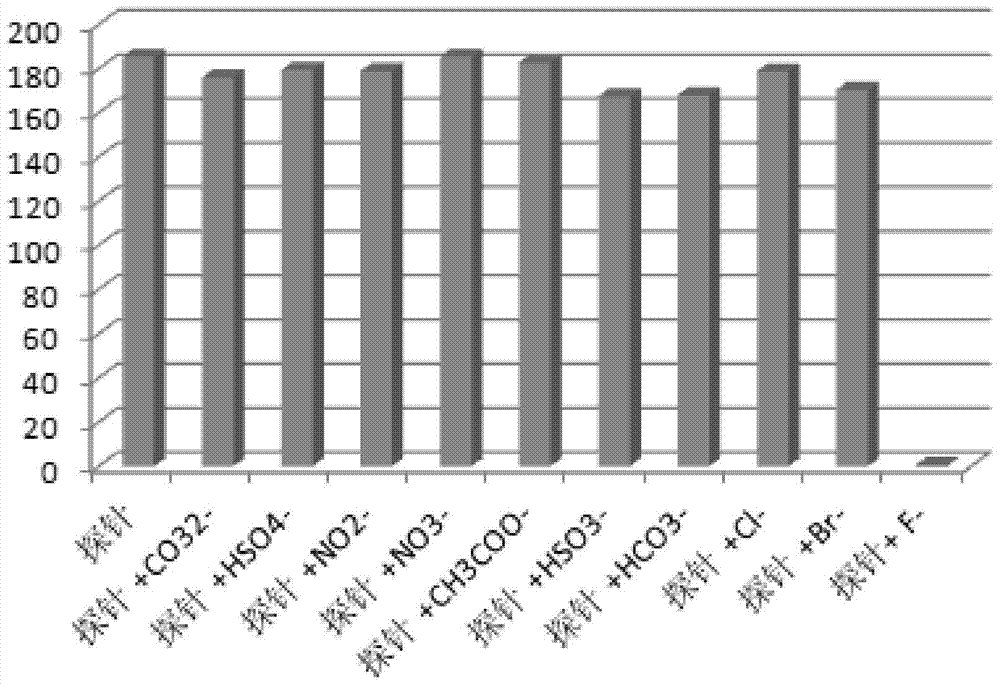
[0063] Example 3: UV-visible spectrum test of the complex’s response to fluoride ions: Prepare a 40 μM complex solution (tetrahydrofuran solvent), pipette 2 mL of the complex solution into a fluorescence cuvette, and gradually add 0-5 eq of pure water dropwise for The fluoride ion solution of the solvent until it reaches equilibrium (that is, the spectrum no longer changes significantly), and the UV-visible emission spectra of no addition and dropwise addition of different contents of semi-fluoride ions are measured, as shown in figure 1 . The test data show that: with the addition of fluoride ions, the absorption peak around 400nm increases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com