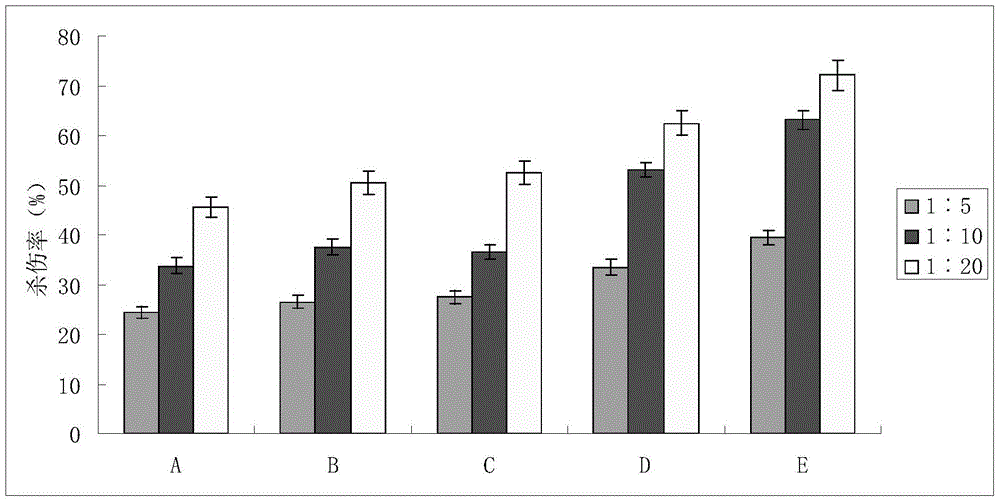
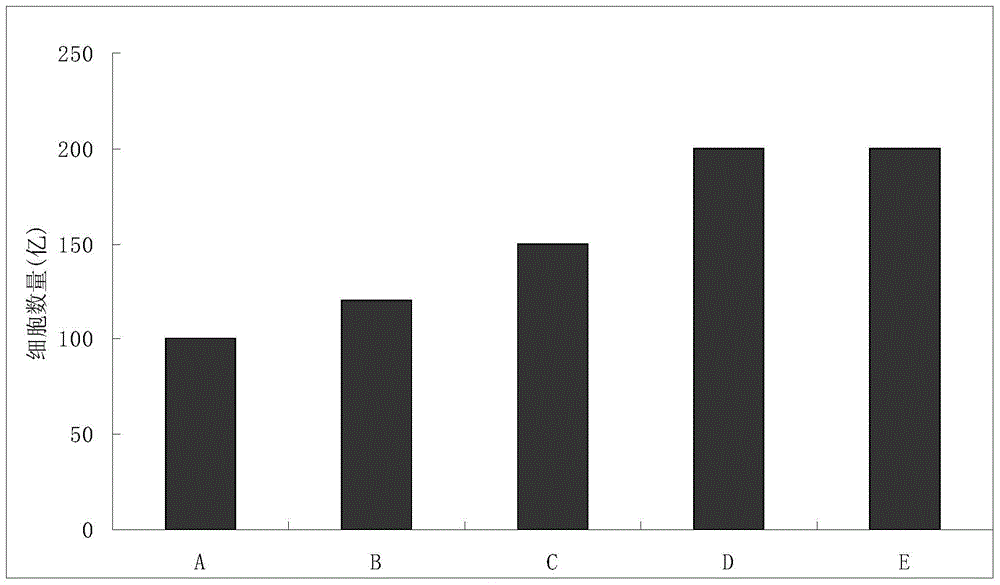
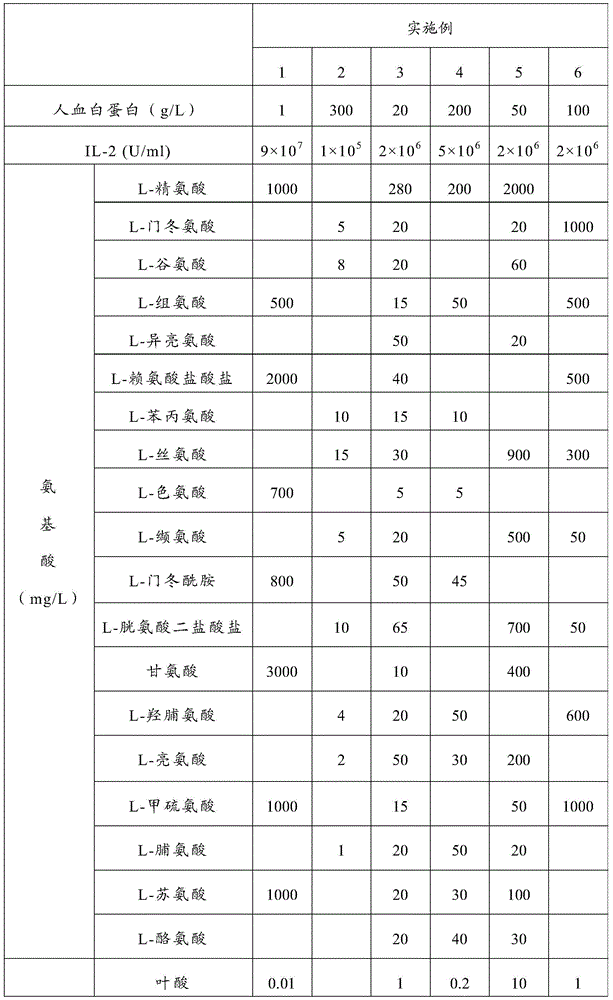
Enhanced cik cell preparation method and cell preparation
A cell preparation, enhanced technology, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problem that immune cells cannot achieve the effect of killing tumors, CIK cell proliferation times and cytotoxic activity are not ideal, etc. problem, to achieve the effect of increasing the killing effect and increasing the toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for preparing enhanced CIK cells, comprising the following steps:
[0041] 1) Collect the patient’s anticoagulant blood under sterile conditions, centrifuge at 1500rpm / min for 6 minutes to absorb the upper plasma, inactivate at 56°C for 30 minutes and then centrifuge for use. Dilute the blood cell pellet with compound normal saline with the same volume as the blood cell pellet, with a specific gravity of 1.077 Human lymphocyte separation solution and diluted blood were added to the centrifuge tube at a ratio of 1:1.5, centrifuged at 2000rpm / min for 15 minutes, carefully extracted the white blood cell layer in the middle, washed twice with compound normal saline, and obtained peripheral blood mononuclear cells after low-speed centrifugation (PBMC);
[0042] 2) Peripheral blood mononuclear cells were isolated from peripheral blood collected from patients; cultured in blood-free medium to obtain 1×10 6 nucleated cells / ml, add IFN-γ, phytohemagglutinin and PGE2, a...
Embodiment 2
[0062] A method for preparing enhanced CIK cells, comprising the following steps:
[0063] 1) Collect the patient’s anticoagulant blood under sterile conditions, centrifuge at 1500rpm / min for 6 minutes to absorb the upper plasma, inactivate at 56°C for 30 minutes and then centrifuge for use. Dilute the blood cell pellet with compound normal saline with the same volume as the blood cell pellet, with a specific gravity of 1.077 Human lymphocyte separation solution and diluted blood were added to the centrifuge tube at a ratio of 1:1.5, centrifuged at 2000rpm / min for 15 minutes, carefully extracted the white blood cell layer in the middle, washed twice with compound normal saline, and obtained peripheral blood mononuclear cells after low-speed centrifugation (PBMC);
[0064] 2) Peripheral blood mononuclear cells were isolated from peripheral blood collected from patients; cultured in blood-free medium to obtain 1×10 6 nucleated cells / ml, add IFN-γ, phytohemagglutinin and PGE2, a...
Embodiment 3
[0069] A method for preparing enhanced CIK cells, comprising the following steps:
[0070] 1) Collect the patient’s anticoagulant blood under sterile conditions, centrifuge at 1500rpm / min for 6 minutes to absorb the upper plasma, inactivate at 56°C for 30 minutes and then centrifuge for use. Dilute the blood cell pellet with compound normal saline with the same volume as the blood cell pellet, with a specific gravity of 1.077 Human lymphocyte separation solution and diluted blood were added to the centrifuge tube at a ratio of 1:1.5, centrifuged at 2000rpm / min for 15 minutes, carefully extracted the white blood cell layer in the middle, washed twice with compound normal saline, and obtained peripheral blood mononuclear cells after low-speed centrifugation (PBMC);
[0071] 2) Peripheral blood mononuclear cells were isolated from peripheral blood collected from patients; cultured in blood-free medium to obtain 1×10 6 nucleated cells / ml, add IFN-γ, phytohemagglutinin and PGE2, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com