Composition for stimulating and inducing single karyocyte to be amplified to gamma deltaT cell and application of composition
A nuclear cell and composition technology, applied in the direction of animal cells, vertebrate cells, cell culture active agents, etc., can solve the problems of low amplification factor, low cell purity, low content of γδT cells, etc., and achieve high proliferation ability, The effect of high purity and high cytotoxic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
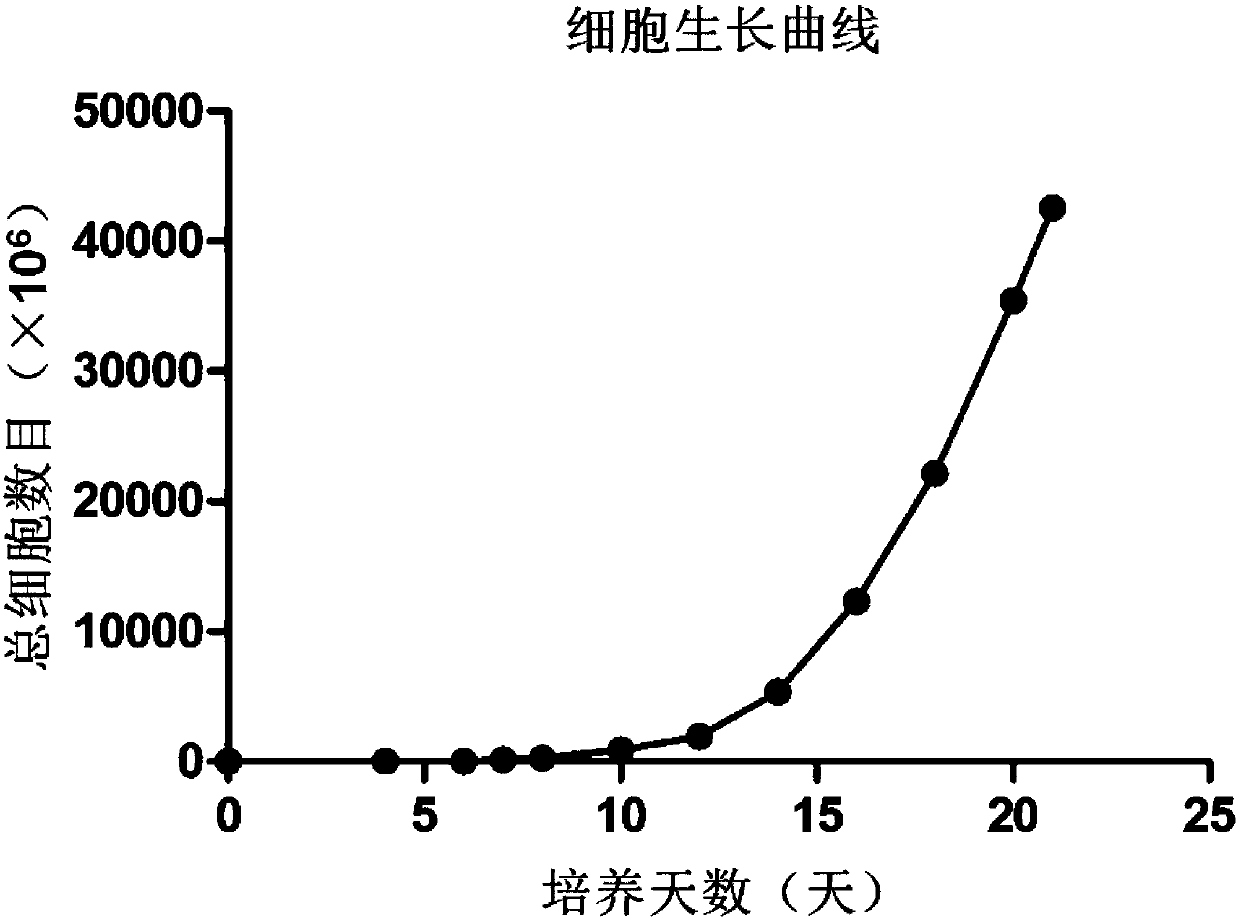
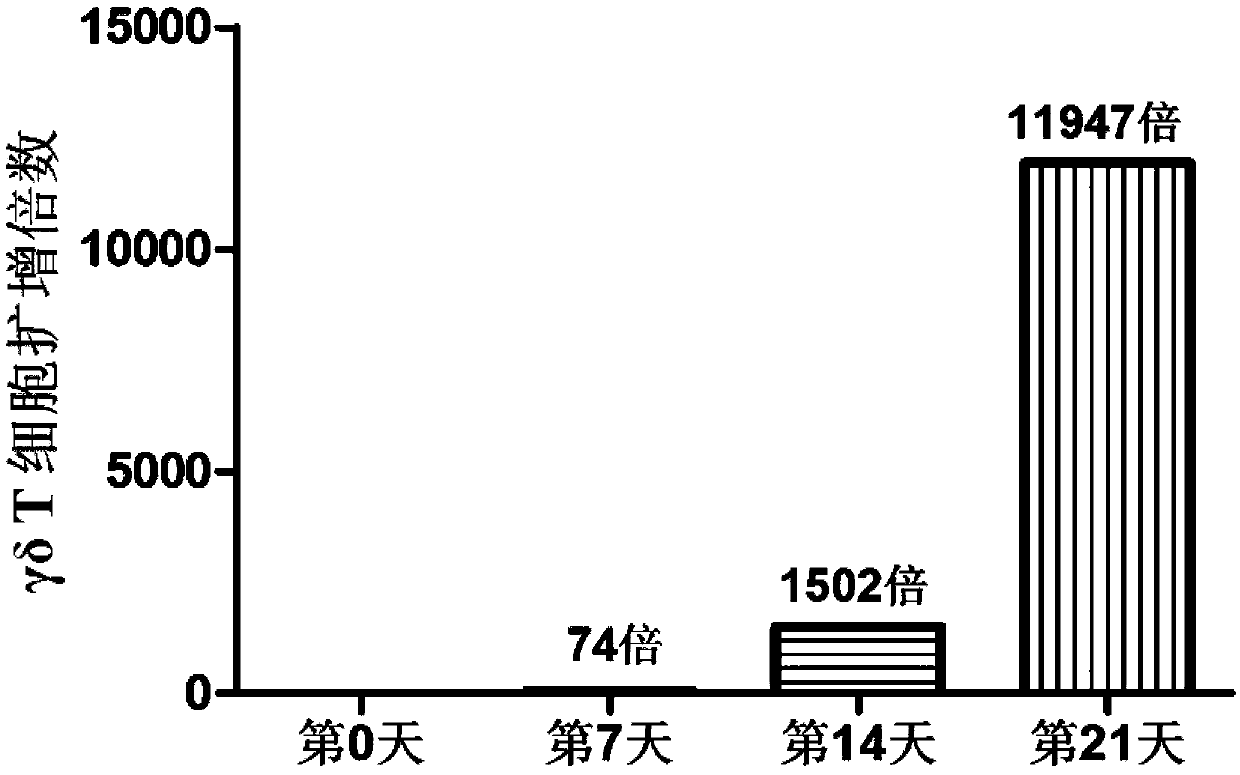
[0030] Example 1: Induction of γδT cells
[0031] Isolation of mononuclear cells (PBMCs) from peripheral blood and expansion of γδ T cells:
[0032] ① Turn on the biological safety cabinet 30 minutes before use;
[0033] ②Take D-PBS out of the refrigerator before use, and let it stand at room temperature for 30 minutes;
[0034] ③Transfer 30ml of peripheral blood samples (heparin anticoagulant) to two sterile 50ml centrifuge tubes, 15ml in each tube, then add 22.5ml of sterile D-PBS to each tube, invert the centrifuge tubes repeatedly, and mix well;
[0035] ④ Take two 50ml sterile centrifuge tubes, add 15ml Ficoll-Paque Plus solution respectively, and then slowly add 24ml blood diluted in step 3 (drawn from the two sterile tubes in step 3) to form layers , 20°C, 400×g, centrifuge for 30 minutes;
[0036] ⑤Put the two 50ml centrifuge tubes in step 4 into a biological safety cabinet, then use a 10ml pipette to suck off the 15ml serum layer, put it into a new sterile 50ml cen...
Embodiment 2
[0045] Embodiment 2: culture medium change liquid
[0046] ①Put the mixed culture medium in a 37°C water bath to warm it up, or put it at room temperature to equilibrate for 1 hour to room temperature;
[0047] ②Put the cell culture bottle into the biosafety cabinet, resuspend the cells, blow evenly with a 25ml pipette, and take 10μl of the cell suspension for counting;
[0048] ③ On day 4, transfer all the cells into a 50ml sterile centrifuge tube and replace the expansion medium. The specific replacement operation is as follows:
[0049] ④According to the total amount of cells, adjust the final cell concentration to 1×10 6 cells / ml. Among them, the improved method is: take half of the new mixed culture composition containing the expansion factors (anti-human CD3Ab, anti-human CD28Ab, IL-15, IL-21, and IL-2) required for amplification by the improved method The base is added to an empty cell culture bottle; the routine culture method is: take half of the new RPMI 1640 medi...
Embodiment 3
[0060] Example 3: Phenotype detection of γδT cells expanded and cultured by improved culture method
[0061] ①Take the cells cultured on the 21st day and put them into two 1.5mL EP tubes, each with 1 × 10 6 cells, centrifuge at 400×g for 5 minutes, and discard the supernatant;
[0062] ②Add 1 mL of LPBS to wash again, centrifuge at 400×g for 5 minutes, and remove the supernatant;
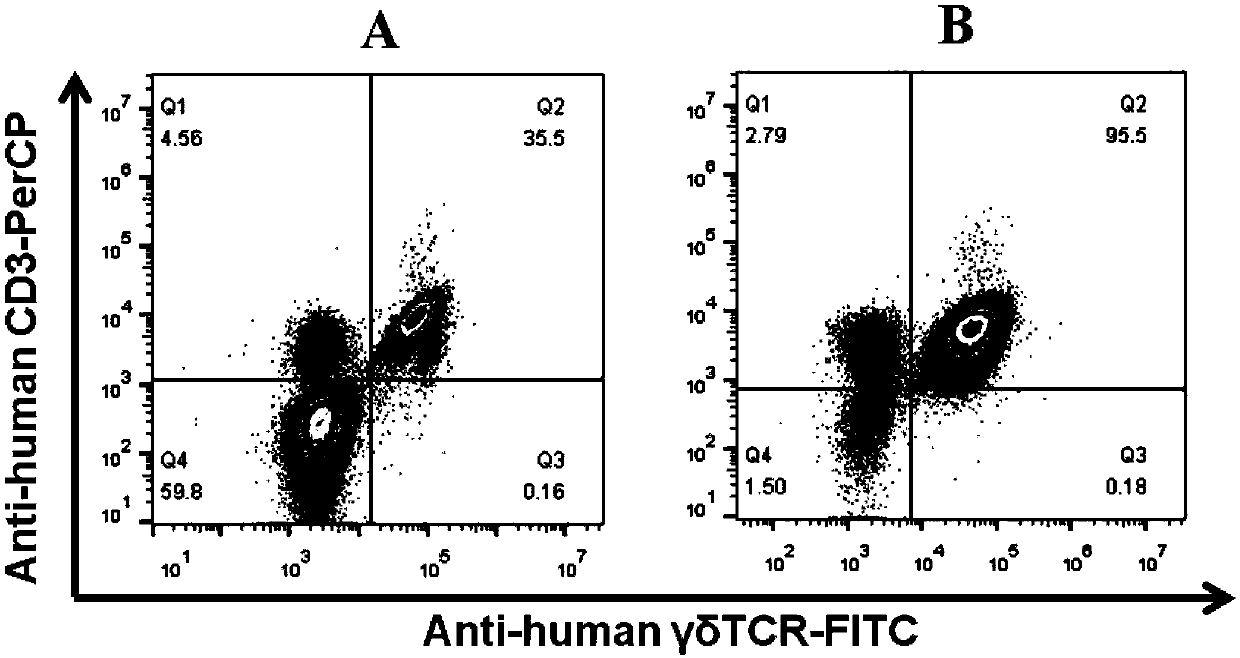
[0063] ③ Add 100 μL of PBS, and then add control antibodies Mouse IgG1-PerCP, Mouse IgG1-FITC ( Figure 4 Middle Ctrl) and detection antibody mouse anti-human CD3-PerCP, anti-human γδTCR-FITC fluorescent antibody ( Figure 4 Medium CD3 / γδ-TCR), placed at 4°C for 30 minutes;
[0064] ④Wash twice with PBS, discard the supernatant, and finally resuspend the cells with 200 μL PBS.
[0065] Use the AECA Novocyte flow cytometer to detect the cultured γδT cells, such as Figure 4 It was shown that the purity of γδT cells can reach 97.8% after culturing the cells for 21 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com