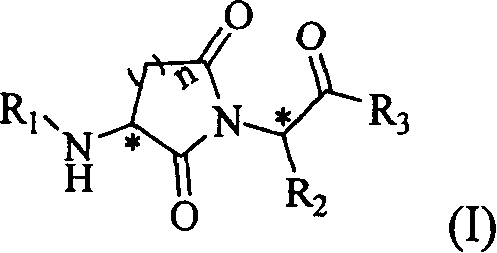
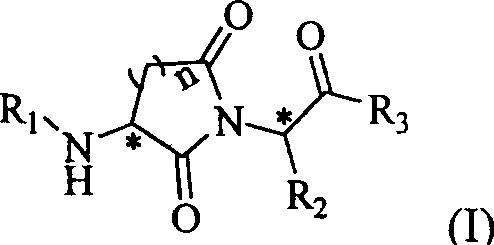
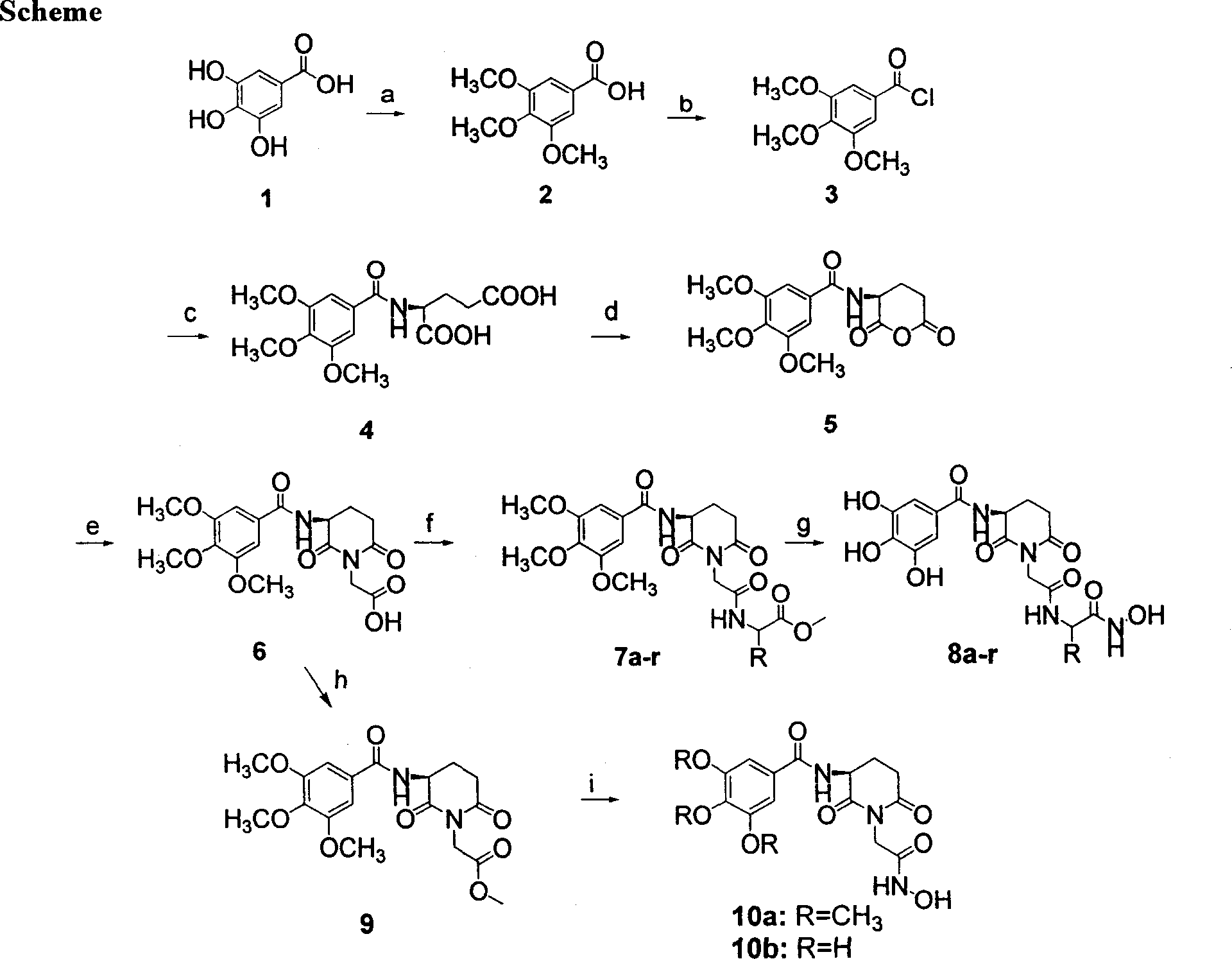
Cyclin imide peptidyl metalloprotease inhibitor and its application
A peptide compound, trimethoxyaniline-based technology, used in cyclic imide peptide metalloproteinase inhibitors and their application fields, can solve the problem of relatively sensitive enzymatic degradation, poor member selectivity, and weakened recognition and killing of tumor cells. abilities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1. Preparation of (S)-2-(3,4,5-trimethoxyanilino)glutaric acid (4)
[0094] 1) 3,4,5-Trimethoxybenzoic acid (2). Dissolve NaOH (80g, 2mol) in 500ml distilled water, cool to room temperature, add 3,4,5-trihydroxybenzoic acid (gallic acid, 50g, 0.294mol), immediately stopper, stir until dissolved. Control the internal temperature below 20°C, add (CH 3 ) 2 SO 4 (89g, 67ml, 0.71mol), at this time the temperature can rise to 30-45°C, open the cork from time to time to deflate. After 20min, add the second batch of equal amount of (CH 3 ) 2 SO 4 At this time, the temperature can rise to 40-45°C, stir for 10 minutes, heat up and boil for 2 hours. Then add 50g of 40% NaOH solution and boil for 2h to saponify the ester that may be generated. The reaction solution was cooled to room temperature, acidified with dilute hydrochloric acid, filtered with suction, and the filter cake was washed with cold water to obtain 48 g of crude product.
[0095] Crude refinement. ...
Embodiment 2
[0098] Example 2. Preparation of (S)-2-(2,6-dioxo-3-(3,4,5-trimethoxybenzamido)piperidin-1-yl)acetic acid (6)
[0099] 1) (S)-N-(2,6-Dioxy-tetrahydro-2H-pyran-3-yl)-3,4,5-trimethoxybenzamide (5). 3,4,5-trimethoxybenzoylglutamic acid (10g, 2.9mmol), add 80ml of acetic anhydride, and keep warm in an oil bath at 55-60°C for 5h. Remove the insoluble matter by filtration while hot, add an appropriate amount of anhydrous ether, cool, and precipitate a white solid. 5.2 g was weighed by filtration, yield 55%, mp 150-152°C. IR (KBr, cm-1): 3310.0, 2945.1, 1777.0, 1640.7, 1504.2, 1239.6, 1129.6; ESI-MS: m / z 323.8.
[0100] 2) (S)-2-(2,6-Dioxy-3-(3,4,5-trimethoxybenzamido)piperidin-1-yl)acetic acid (6). Compound 5 (5.0 g) was dissolved in 50 ml of DMF, 1.0 g of glycine was ground and added to DMF, and heated at 110° C. for 5 hours. The mixture was cooled to room temperature, poured into an equal volume of ice water with a pH of 2, and left overnight, a large amount of white crystals ...
Embodiment 3
[0101] Example 3. (S)-Methyl-2-(2-(2,6-dioxo-3-(3,4,5-trimethoxyanilino)piperidin-1-yl)acetamido) Preparation of Acetate (7a).
[0102] Compound 6 (1.90g, 5mmol) and HoBt (0.81g, 6mmol) were dissolved in 20ml of dichloromethane and 2ml of dimethyl sulfoxide, cooled to 0°C in an ice bath, and slowly added dropwise with EDCI (1.44g, 7.5mmol). Chloromethane solution was added dropwise in about 1 hour, then removed from the ice bath and stirred for 2 hours. Glycine methyl ester hydrochloride (0.75 g, 6 mmol) was added in batches in an ice bath, and the pH value was adjusted to 7 with triethylamine. After the ice cubes melted automatically, the stirring was continued overnight. The reaction liquid was successively washed with 1N hydrochloric acid, 1% sodium carbonate, saturated brine, dried over sodium sulfate, and purified by column chromatography to obtain the final product with a yield of 79%. mp98.7-100.6℃. 1 HNMR (400MHz, DMSO-d6, ppm): δ9.04(t, 1H), 8.88(d, 1H), 7.17(s, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com