Electrolyte of mixed type super capacitor
A technology of supercapacitors and electrolytes, applied in the direction of hybrid capacitor electrolytes, etc., to achieve good charge-discharge cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Electrodes were prepared and button-type lithium titanate / activated carbon hybrid supercapacitors were assembled according to the method described in "Specific Embodiments".
[0023] The composition and ratio (volume) of the electrolyte used are as follows:
[0024] Solvent: 1 part of propylene carbonate (PC), 1 part of ethylene carbonate (EC), 1 part of dimethyl carbonate (DMC);
[0025] Solute: Tetraethylammonium tetrafluoroborate (TEABF 4 ) concentration 0.3mol / L; lithium hexafluorophosphate (LiPF 6 ) concentration of 0.7mol / L.
[0026] The conductivity of the prepared electrolyte was 11.62mS / cm. The results show that the conductivity of the electrolyte prepared in Example 1 is higher than that of the electrolyte in Comparative Example 1 (only lithium hexafluorophosphate is used as the electrolyte salt) (10.96 mS / cm).
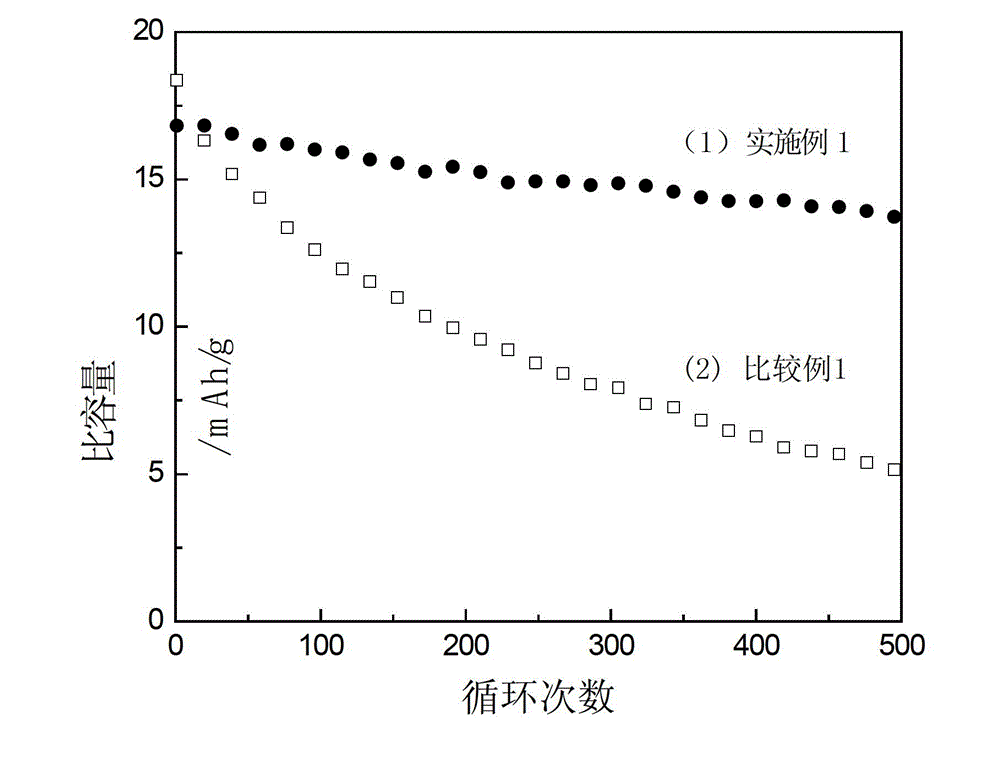
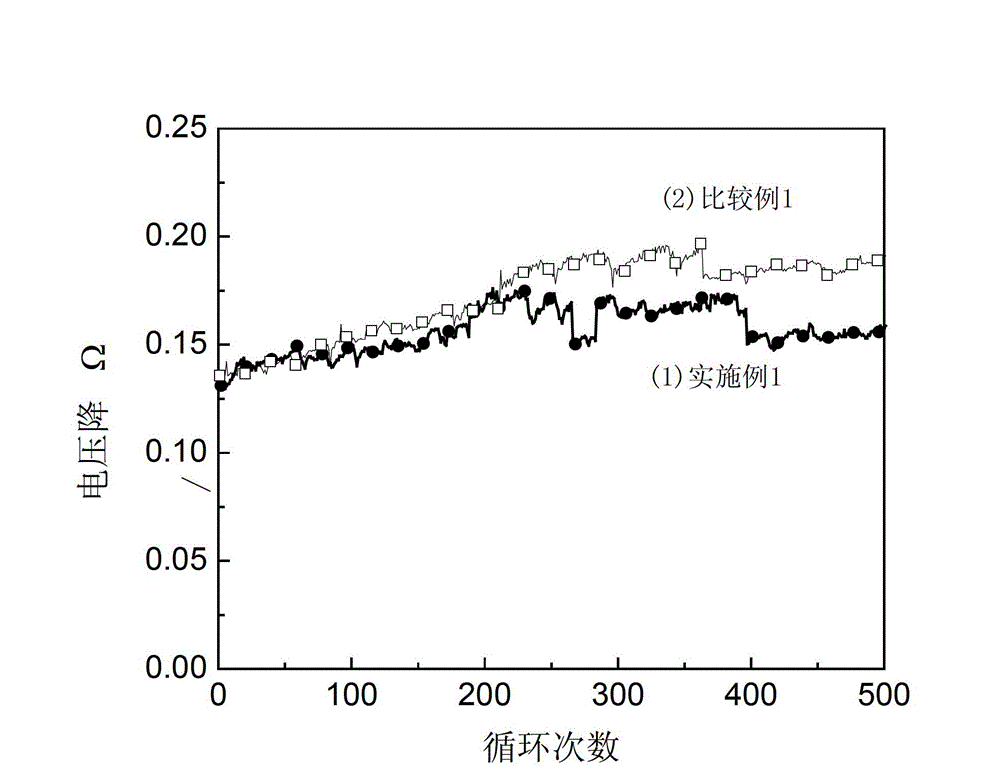
[0027] figure 1 The middle curve (1) shows the change of the specific capacity of the lithium titanate / activated carbon hybrid supercapacitor as...
Embodiment 2
[0030] Prepare electrodes and assemble button-type lithium titanate / activated carbon hybrid supercapacitors according to the method described in "Specific Embodiments".
[0031] The composition and ratio (volume) of the electrolyte used are as follows:
[0032] Solvent: 1 part of propylene carbonate (PC), 1 part of ethylene carbonate (EC), 2 parts of dimethyl carbonate (DMC),
[0033] Solute: Tetraethylammonium tetrafluoroborate (TEABF 4 ) concentration 0.5mol / L; lithium hexafluorophosphate (LiPF 6 ) concentration of 0.5mol / L.
[0034] The conductivity of the prepared electrolyte was 11.47mS / cm. It shows that the conductivity of the electrolyte prepared in Example 2 is higher than that of the electrolyte salt in Comparative Example 1 (10.96 mS / cm).
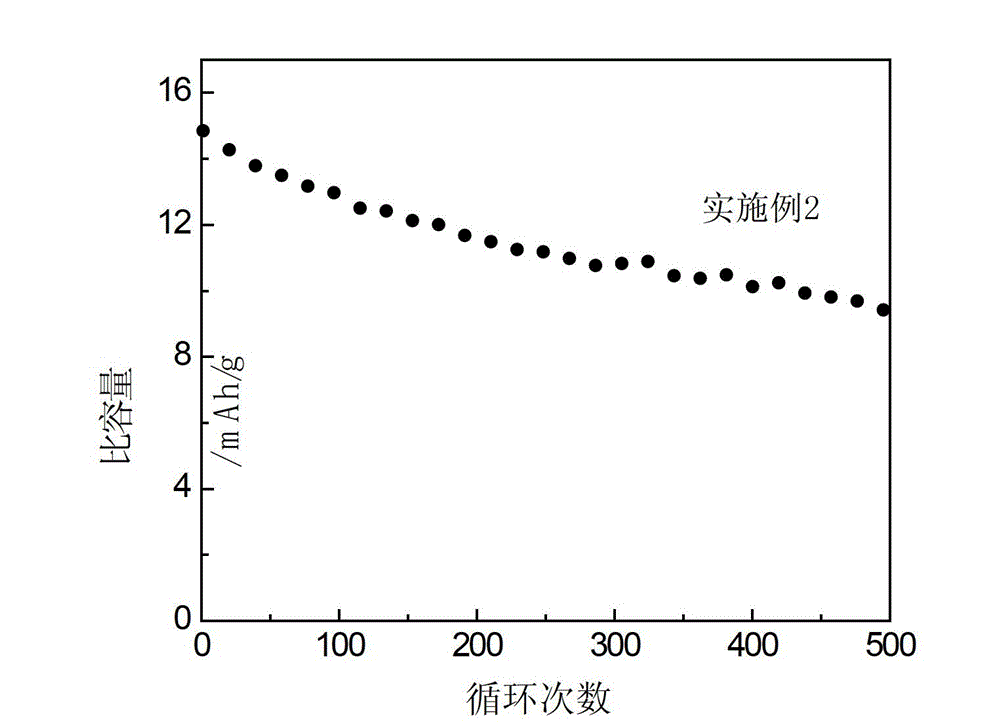
[0035] image 3 The change of the specific capacity of the hybrid supercapacitor using the electrolyte prepared in Example 2 with the number of cycles. The results in the figure show that the cycle stability of the hybrid su...
Embodiment 3
[0037] Prepare electrodes and assemble button-type lithium titanate / activated carbon hybrid supercapacitors according to the method described in "Specific Embodiments".
[0038] The composition and ratio (volume) of the electrolyte used are as follows:
[0039] Solvent: 1 part of propylene carbonate (PC), 1 part of ethylene carbonate (EC), 2 parts of dimethyl carbonate (DMC),
[0040]Solute: Tetraethylammonium tetrafluoroborate (TEABF 4 ) concentration 1.0mol / L; lithium hexafluorophosphate (LiPF 6 ) concentration of 0.5mol / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com