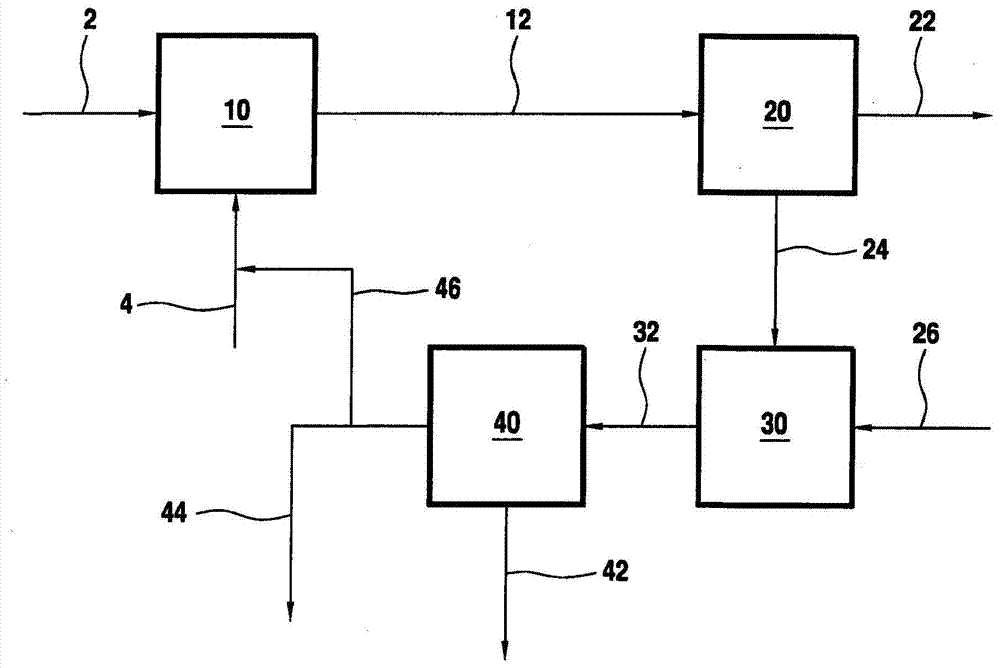
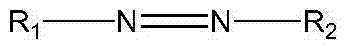
Integrated process for producing polyvinyl alcohol or a copolymer thereof and ethanol
一种共聚物、乙烯醇的技术,应用在化学仪器和方法、有机化合物的制备、羟基化合物制备等方向,能够解决限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The distillation is carried out with the stream from the PVOH process. In the laboratory, an Oldershaw column with 40 trays was used. A stream of mother liquor containing 0.24 wt% solids was fed near the middle of the column, while a stream of aqueous methanol containing 0.13 wt% solids was fed about one-third from the bottom of the column. The temperatures at the top and bottom of the column in atmospheric distillation are 68°C and 100°C, respectively. The feed rate of the mother liquor was 13.7 g / min, and the feed rate of the aqueous methanol solution was 11.5 g / min. The reflux ratio was maintained at about 0.23. No foaming or major fouling problems in the reboiler were observed during the distillation. Dark brown / black staining or fouling was observed around plate 15 to the bottom of the column. However, this slight fouling does not plug the small plate holes or downcomers of the Aldershaw column. The tray above the mother liquor feed is clean.
[0080] Table 1...
Embodiment 2
[0087] Example 2 (estimation)
[0088] This example describes the hydrogenolysis of methyl acetate as reported in paragraph [0064] of US Patent Publication 2009 / 0326080. The methyl acetate was kept as a liquid at 20°C and was pumped at a pressure of 10-50 atm through a heat exchanger which completely vaporized it at a temperature of 150°C-225°C. Hydrogen preheated at the same temperature is added to the steam as it leaves the heat exchanger. h 2 The molar ratio to methyl acetate is 5-10. The hot mixture is blown through a catalyst bed comprising CuO / copper chromite, CuO / ZnO / Al 2 o 3 , or CuO / ZnO / activated carbon catalyst and an inert solid acting as catalyst diluent. Before adding any acetate, by adding H 2 and N 2 The mixture reduces CuO to Cu. This reduces CuO to the active form Cu in the hydrogenolysis reaction. Reduction is carried out until no more water is produced. By adding H in the gas mixture 2 The concentration was kept at a level not exceeding 5 vol%, ...
Embodiment 3
[0090] This example also describes the hydrogenolysis of methyl acetate. exist Six runs were performed in a continuous stirred tank reactor (CSTR). The same amount of 40 mL of copper-zinc oxide on alumina support was used in all 6 experiments, namely (Süd Chemie). The first 4 runs were run at ~360-375 psig, while the last 2 runs were run at a higher pressure of 625 psig. At 360 psig, test two reactions at 250°C, then two at 275°C; one temperature at 250°C at 625 psig. Methyl acetate LHSV at 0.85hr in all 6 trials -1 with 1.25hr -1 Alternate between, H 2 The ratio to methyl acetate remains constant at about 14:1 H 2 molar ratio to methyl acetate.
[0091] Table 2 provides the reaction conditions and results for the six methyl acetate trials. Table 3 provides an overview of the product composition for Experiment 2.
[0092] In Table 2, methyl acetate conversion, methanol selectivity, ethanol selectivity and ethanol yield were calculated.
[0093] with (X 1 -Y 1 )×...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com