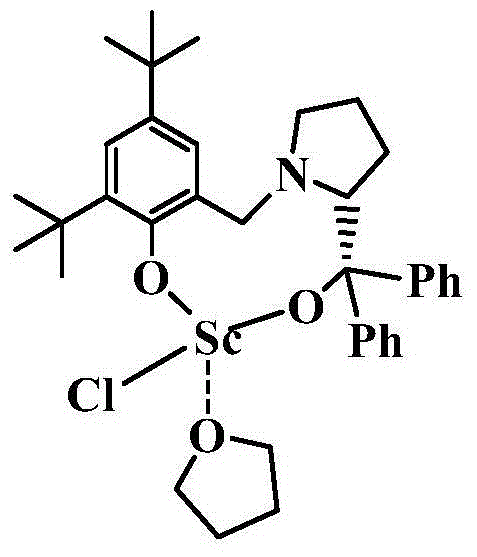
Chiral bridged aryloxyalkyloxy scandium chloride, and preparation method and application thereof
An aryloxyalkoxy scandium and chloride technology, which is applied in the field of asymmetric catalysis, can solve problems that have not yet been seen, and achieves the effects of a wide range of substrate adaptation, a clear compound structure, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of LScCl(THF)
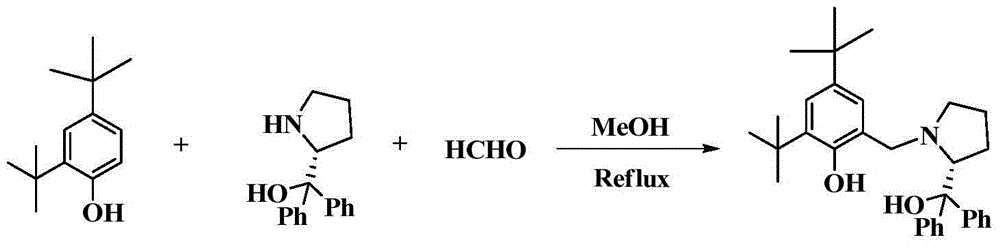
[0050] 1) Before preparing the chiral bridged aryloxyalkoxy scandium compound, first prepare LH 2 , its preparation method is as follows:
[0051] Add 3 ml (30 mmol) of formaldehyde solution into a two-neck flask with stirring bar, add methanol to dissolve, then add (S)-α, 5.07 g (20 mmol) of α-diphenylprolinol, and stir the reaction After half an hour, 4.12 g (20 mmol) of 2,4-di-tert-butylphenol was added. After reacting at 60°C for 24 hours, a large amount of white solid precipitated out. Suction filtration, washing the filter cake with methanol, and drying to obtain the compound LH 2 , yield 80%. NMR data: 1 H NMR (400MHz, CDCl 3 ): 7.60(dd, 4H), 7.32(m, 4H), 7.21(t, 1H), 7.18-7.08(m, 2H), 6.70(s, 1H), 3.99(m, 1H), 3.41(dd, 2H), 2.90(s, 1H), 2.43(m, 1H), 2.15-2.01(m, 1H), 1.96-1.83(m, 1H), 1.70(m, 2H), 1.39(s, 9H), 1.24 (s,9H).
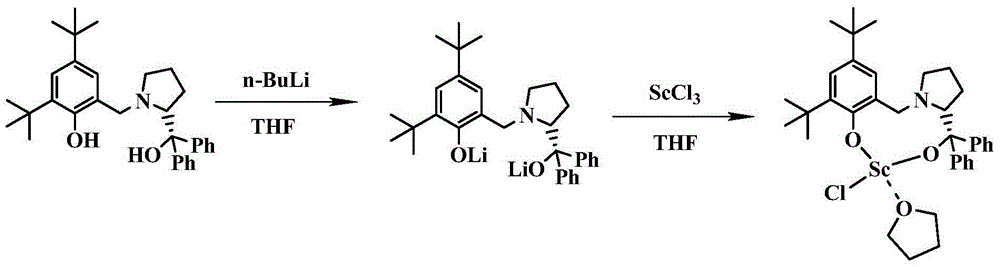
[0052] 2) Preparation of LScCl(THF)
[0053] Add LH to a reaction flask that strictly remo...
Embodiment 2
[0054] Example 2: Using the LScCl (THF) prepared above to catalyze the asymmetric hydrophosphine reaction of α, β-unsaturated ketones:
[0055] In the reaction flask that has been treated with dehydration and deoxygenation, add 0.0625 g (0.3 mmol) of chalcone and 0.019 g (0.03 mmol) of chiral bridged aryloxyalkoxy scandium chloride under the protection of argon, and add 2.0 ml of tetrahydrofuran was stirred in a constant temperature bath with a set temperature of 10°C for 5 minutes, and then 0.058 ml of diethyl phosphinate was added. After continuing the reaction at 10° C. for 4 h, the reaction was terminated with deionized water.
[0056] The product was separated on a silica gel column, and finally eluted with an eluent of ethyl acetate:petroleum ether=1:1 to obtain 45.72 mg of α-hydroxyphosphonate with a yield of 44%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak AS-H column, eluent i-PrOH / hexane (10 / 90), flow rate 1.0mL / min, ee value 37%.
Embodiment 3
[0057] Example 3: Using the LScCl (THF) prepared above to catalyze the asymmetric hydrophosphine reaction of α, β-unsaturated ketones:
[0058] In the reaction flask that has been treated with dehydration and deoxygenation, add 0.0625 g (0.3 mmol) of chalcone and 0.019 g (0.03 mmol) of chiral bridged aryloxyalkoxy scandium chloride under the protection of argon, and add 2.0 ml of toluene was stirred in a constant temperature bath with a set temperature of 10° C. for 5 minutes, and then 0.058 ml of diethyl phosphinate was added. After continuing the reaction at 10° C. for 4 h, the reaction was terminated with deionized water.
[0059]The product was separated on a silica gel column, and finally washed with an eluent of ethyl acetate:petroleum ether=1:1 to obtain 56.11 mg of α-hydroxyphosphonate with a yield of 54%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak AS-H column, eluent i-PrOH / hexane (10 / 90), flow rate 1.0mL / min, ee value 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com